Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av pasienter med spiserørskreft
Forord
Sist faglig oppdatert: 28.06.2022
Mange medisinske faggrupper hadde i en årrekke lagt ned et betydelig arbeid for å komme frem til konsensusbaserte faglige anbefalinger for diagnostikk og behandling av ulike typer kreft. Som ledd i Nasjonal strategi for kreftområdet (2006–2009), fikk Helsedirektoratet i oppdrag å videreutvikle og oppdatere faggruppenes anbefalinger til nasjonale handlingsprogrammer for kreftbehandling, i nært samarbeid med fagmiljøene, de regionale helseforetakene, Nasjonalt kunnskapssenter for helsetjenesten, og andre relevante myndigheter.
Nasjonale handlingsprogrammer for kreft skal bidra til at det offentlige tilbudet i kreftomsorgen blir av god kvalitet og likeverdig over hele landet. Målgrupper for retningslinjene er leger og legespesialister innen medisin, kirurgi, onkologi, radiologi, patologi og fastleger. De vil også være av interesse for andre faggrupper som er involvert i behandling og oppfølging av kreftpasienter og deres pårørende.
Nasjonale faglige retningslinjer fra Helsedirektoratet er å betrakte som anbefalinger og råd, basert på oppdatert faglig kunnskap som er fremskaffet på en systematisk, kunnskapsbasert måte. De nasjonale faglige retningslinjene gir uttrykk for hva som anses som god praksis på utgivelsestidspunktet, og er ment som et hjelpemiddel ved de avveininger tjenesteyterne må gjøre for å oppnå forvarlighet og god kvalitet i tjenesten. Nasjonale faglige retningslinjer er ikke direkte rettslig bindende for mottagerne, men bør langt på vei være styrende for de valg som skal tas. Ved å følge oppdaterte nasjonale faglige retningslinjer vil fagpersonell bidra til å oppfylle kravet om faglig forsvarlighet. Dersom en velger løsninger som i vesentlig grad avviker fra de nasjonale faglige retningslinjene, bør en dokumentere dette, og være forberedt på å begrunne sine valg. Sykehusenes eiere og ledelse bør tilrettelegge virksomheten slik at de nasjonale faglige retningslinjene kan følges.
Helsedirektoratet takker arbeidsgruppen for stor innsats i utarbeidelsen av handlingsprogrammet. Vi håper handlingsprogrammet vil være et nyttig arbeidsredskap ved behandling av pasienter med spiserørskreft. Innholdet i den nasjonale retningslinjen for spiserørskreft vil vurderes årlig, og om nødvendig oppdateres. Disse nasjonale faglige retningslinjene for diagnostikk, behandling og oppfølging av pasienter med spiserørskreft er publisert 28.06.2022.
Bjørn Guldvog
Helsedirektør
Innledning
Sist faglig oppdatert: 28.06.2022
Nytt i denne utgaven av Handlingsprogrammet for spiserørkreft
- Handlingsprogrammet er oppdatert med de senest tilgjengelige data fra Kreft og Kvalitetsregister
- Histopatologisk vurdering er mer utførlig presentert, inkludert omtale av molekylær undersøkelse
- Thorakolaparoskopiske teknikker og evidensgrunnlaget for valg av tilgang er utførlig omtalt
- Det foreligger en omfattende omtale av lymfeknutemetastasering og lymfadenektomi. Dette inkluderer en oppsummering av tilgjengelig evidens og en anbefaling omkring omfang av lymfadenektomi.
- Det foreligger en mer utførlig omtale av både perioperativ og palliativ stråle og/eller kjemoterapi. Dette inkluderer nyere behandlingsformer som immunterapi samt krav til differensiert behandling.
Sammendrag av retningslinjene
Sist faglig oppdatert: 28.06.2022
|
| Kunnskapsgrunnlagets evidensgrad |
|---|---|
| UTREDNING |
|
| Pasienter med resektabel spiserørskreft bør behandles ved sentra med spesiell kompetanse og evalueres av multidisiplinære team. | C |
| Pasienter med nyoppstått dysfagi skal snarest henvises til øvre endoskopi. | D |
| Ved påvist spiserørskreft tas CT av hals, thorax og abdomen (helst som dedisert spesialprosedyre). | B |
| EUS anbefales for stadieinndeling når dette kan få terapeutiske konsekvenser. | A |
| MR er ikke indisert rutinemessig. | A |
| PET-CT anbefales for deteksjon av fjernmetastaser når dette kan få terapeutiske konsekvenser. | B |
| BEHANDLING AV DYSPLASI OG KREFT I TIDLIG STADIUM |
|
| Pasienter med dysplasi og intramukosalt karsinom bør få tilbud om endoskopisk behandling. | C |
| Endoskopisk behandling bør sentraliseres til få sykehus. | D |
| KIRURGISK OG ONKOLOGISK BEHANDLING MED KURATIV INTENSJON |
|
| Ved svulst i spiserøret anbefales minimum to-felts lymfeknutedisseksjon. | B |
| Pasienter med distal spiserørskreft som tåler større kirurgiske inngrep, bør få utført transtorakal (åpen, hybrid eller minimalt invasiv) reseksjon. | B |
| Etter reseksjon av spiserøret bør pasientene få tidlig enteral ernæring. | B |
| For lokalavansert sykdom (cT2–4 eller cN1–3 M0) anbefales neoadjuvant radiokjemoterapi. |
|
| For adenokarsinomer er perioperativ kjemoterapi med FLOT-regimet et alternativ til preoperativ radiokjemoterapi. |
|
| For pasienter operert for kreft i spiserør eller gastroøsofageale overgang, etter neoadjuvant radiokjemoterapi og som ikke har oppnådd pCR i resektatet, kan adjuvant behandling i inntil 1 år med PD-1 hemmeren nivolumab vurderes. |
|
| Definitiv radiokjemoterapi kan tilbys pasienter med lokalavansert ikke-resektabel spiserørskreft, medisinsk inoperable pasienter som vurderes å tolerere behandlingen, og pasienter som ikke ønsker operasjon. | A |
| Ved svulster i cervikale spiserøret kan man ut ifra en individuell vurdering av pasientens toleranse vurdere strålebehandling til totaldose 60–66 Gy. Ved svulster i distale del av spiserøret og gastroøsofageal overgang anbefales det ikke å overstige totaldose på 50,4 Gy. | A |
| OPPFØLGING OG KONTROLL ETTER AVSLUTTET KURATIV BEHANDLING |
|
| Kontroll etter kurativ behandling er viktig for å sikre adekvat ernæringsfunksjon. | D |
| Ved komplett patologisk respons hos pasienter som har gjennomgått definitiv radiokjemoterapi, kan utvalgte pasienter være aktuelle for salvage kirurgi ved residiv. |
|
| Pasienter som har gjennomgått endoskopisk behandling for dysplasi eller kreft i tidlig stadium skal kontrolleres ved behandlende avdeling. | D |
| Etter endoskopisk behandling bør kontroll med gastroskopi foretas hver 3. måned i ett år, deretter årlig. | D |
| BEHANDLING AV METASTASERENDE SYKDOM/LIVSFORLENGENDE OG PALLIATIV BEHANDLING |
|
| Pasienter i god almenntilstand og WHO 0–2 bør vurderes for cytostatikabehandling ev. i kombinasjon med immunterapi. | A |
| Førstelinjebehandling: Det finnes ikke ett kjemoterapi-regime som er å foretrekke framfor de andre. Som hovedregel bør et tostoffs regime med et fluoropyrimidin i kombinasjon med oxaliplatin, irinotecan (ved adenokarsinom) eller et taxan vurderes. Ved HER2-positiv adenokarsinom (IHC 2+ og ISH+, eller IHC 3+) anbefales trastuzumab i kombinasjon med platinum og kapecitabin/5FU. Ved PEK med TPS ≥ 1 % anbefales nivolumab + kjemoterapi, alternativt nivolumab i kombinasjon med ipiliumab. Ved PEK med CPS ≥ 10 anbefales pembrolizumab+ kjemoterapi, alternativt nivolumab + kjemoterapi, alternativt nivolumab i kombinasjon med ipiliumab. Ved AK og CPS ≥ 5 anbefales nivolumab + kjemoterapi. Ved AK og CPS ≥ 10 anbefales pembrolizumab + kjemoterapi eller nivolumab + kjemoterapi. Ved MSI-pos/dMMR karsinom anbefales Pembrolizumab eller nivolumab som monoterapi eller som kombinasjonbehandling med kjemoterapi. |
|
| Andrelinje og senere behandling: Taksaner (paclitaxel eller docetaxel) hvis ikke brukt før eller irinotecan basert (FOLFIRI, FLIRI eller irinotekan monoterapi). Tredjelinje/fjerde linje kjemoterapi med trifluridin / tipiracil for AK kan vurderes for pasienter med ECOG 0–1. Pasienter som er ECOG 0-1(2) og PD-L1 CPS ≥ 10, MSI eller EBV positive og som enda ikke har fått behandling med PD-1 hemmer, kan immunterapi med PD-1 hemmer vurderes, etter progresjon på kjemoterapi. |
|
| For pasienter over 75 år eller yngre pasienter med redusert almenntilstand anbefales FLV, capecitabin monoterapi, irinotecan monoterapi, taksaner, evt FLOX/FOLFOX i redusert dose-regimet. | D |
Epidemiologi
Forekomst
Sist faglig oppdatert: 28.06.2022
Spiserørskreft er den 8. hyppiste kreftformen og den 6. vanligste årsak til kreftrelatert død på verdensbasis (Ferlay et al., 2020).
Basert på tall fra Kreftregisteret ble det i 2020 diagnostisert 388 nye tilfeller av spiserørskreft (Cancer Registry of Norway, 2021). Det er verdt å merke at tallene fra Kvalitetsregister for kreft spiserør og magesekk har noe høyere insidenstall for spiserørskreft (486 nye tilfeller i 2020). Årsaken til denne diskrepansen er at pasienten som har kreft i overgangen mellom spiserør og magesekk (ICD-10 C16.0) er inkludert som magesekkreft i Kreftregisterets statistikk, men inkluderes som spiserørskreft i Kvalitetsregisterets analyser.
Insidensen er svakt stigende, og har økt med over 20 % siden begynnelsen av 2000-tallet . Gjennomsnittlig alder ved påvisning av spiserørskreft er 70 år, og i Norge er kreftformen tre ganger hyppigere hos menn enn hos kvinner (Cancer Registry of Norway, 2021; Norway, 2015). De to hovedformene av spiserørskreft er plateepitelkarsinom og adenokarsinom, med en klar overvekt av adenokarsinom (ca 4/5) i Norge, Vest-Europa og Nord-Amerika. Plateepitelkarsinom er hyppigst i cervikale og øvre thorakale del av spiserøret, adenokarsinom i distale del.
Plateepitelkarsinom utgår fra slimhinnen i spiserøret og tobakk og alkohol (brennevin) (Lagergren, Bergstrom, Lindgren, & Nyren, 2000), gir økt risiko for denne sykdommen. Antall nye tilfeller har sunket jevnt gjennom de siste tiår. Fordi risikofaktorene er de samme som for plateepitelkarsinom i øre-nese-halsregionen (ØNH), forekommer en del tilfeller med samtidig (synkron) eller etterfølgende (metakron) spiserørskreft og kreft i ØNH-området.
Adenokarsinom i spiserøret er den kreftformen som prosentvis øker mest i verden, og en antar at dette er relatert til økende overvekt og gastroøsofageal refluks (Lagergren, Bergstrom, Lindgren, & Nyren, 1999; Lagergren, Bergstrom, & Nyren, 1999). Langvarig refluks kan føre til intestinal metaplasi av stamcellene i de basale lagene av plateepitelet i distale spiserør (Barretts øsofagus). Dette er en premalign tilstand, med årlig insidens for utvikling av kreft fra 0,3–0,6 % (de Jonge, van Blankenstein, Grady, & Kuipers, 2014; Sikkema, de Jonge, Steyerberg, & Kuipers, 2010). Hvorvidt alle tilfeller av adenokarsinom er assosiert med Barretts øsofagus er ikke sikkert avklart (Chandrasoma, Wickramasinghe, Ma, & DeMeester, 2007). Frekvens, varighet og alvorlighetsgrad av refluks er assosiert med økende forekomst av Barretts øsofagus. Selv om overvekt er assosiert med refluks, er også overvekt i seg selv en risikofaktor for adenokarsinom i spiserøret (Lagergren, Bergstrom, & Nyren, 1999; World Cancer Research Fund/American Institute for Cancer Research, 2018a). Det er betydelig overvekt av menn ved plateepitelkarsinom i spiserøret (6–8:1). For adenokarsinom vil kjønnsfordelingen nærme seg den som foreligger ved kreft i magesekk (1.3:1). Adenokarsinom er ikke assosiert med alkohol og relativt moderat assosiert med røyking (Wu, Wan, & Bernstein, 2001). Det er funnet en invers sammenheng mellom Helicobacter pylori-infeksjon type Cag A og adenokarsinom i spiserøret (Islami & Kamangar, 2008).
Strålebehandling for annen kreft i overkroppen øker risikoen for senere utvikling av karsinom i spiserøret (Y. Zhang, 2013).
Adenokarsinom utgjør 75–80 % av all spiserørskreft i Norge (Nasjonalt kvalitetsregister for kreft i spiserør og magesekk, 2017).
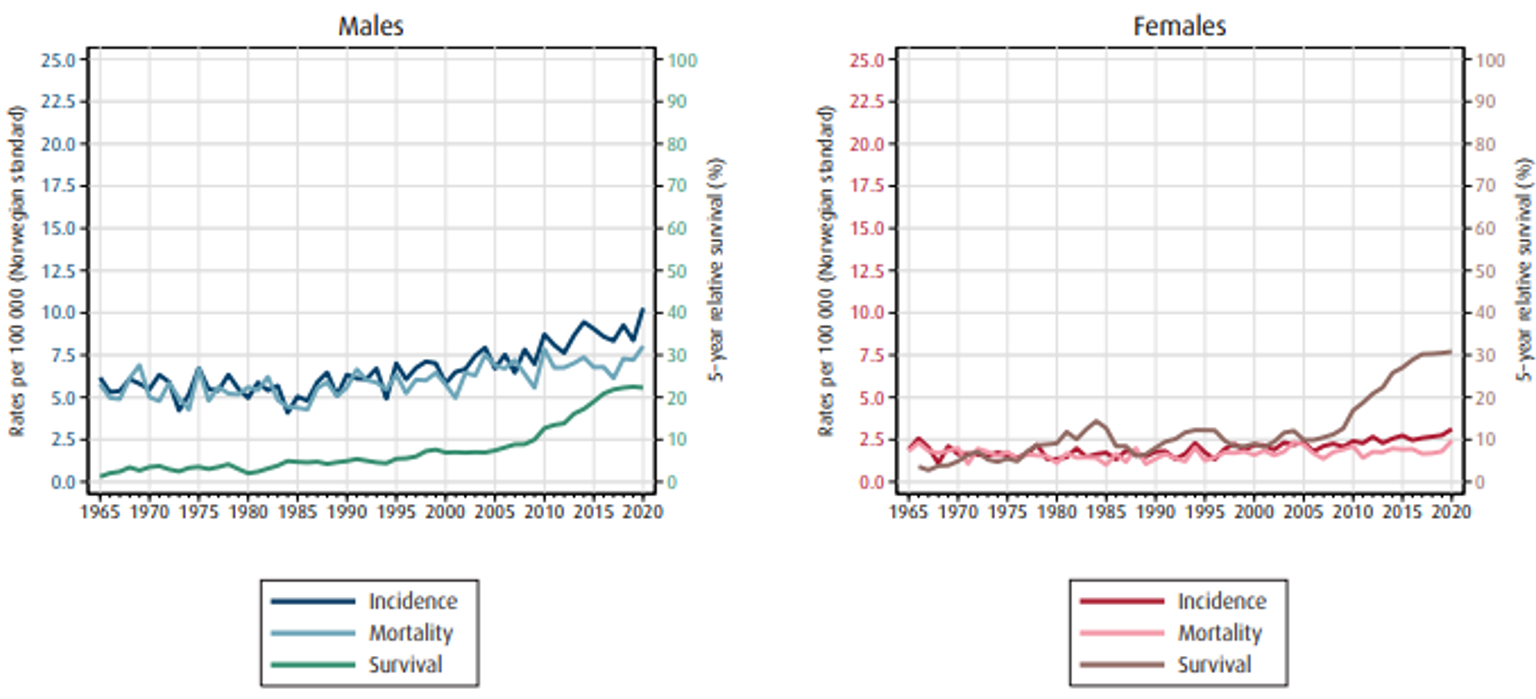
Insidens, mortalitet og relativ overlevelse av spiserørskreft i Norge er vist i figur 1.1 (Cancer Registry of Norway, 2021).
Overlevelse
Sist faglig oppdatert: 28.06.2022
De fleste tilfellene av spiserørskreft, uansett histologi, blir diagnostisert i avansert stadium, og prognosen er totalt sett dårlig. Spiserøret har en mediastinal beliggenhet omgitt av løst bindevev og med manglende serosa, med begrenset barrierefunksjon mot spredning. Dette, sammen med langsgående/longitudinelt forløpende intramuralt lymfatisk nettverk, hvor kreft kan spre seg langs spiserørsveggen, under intakt mukosa. Dette igjen, medvirker til høy sannsynlighet for lymfatisk spredning og fjernmetastaser som begrenser muligheten for kurasjon ved lokoregional terapi alene.
Ved diagnose i perioden 2016–20 hadde 11 % lokalisert sykdom, 33 % regional sykdom, 24 % fjernmetastaser og 32 % hadde ukjent stadium (Upubliserte tall fra kvalitetsregisteret). Ca. 7/10 av pasientene er inoperabele på grunn av avansert sykdom og komorbiditet. Selv om overlevelsen av denne sykdommen fremdeles er lav, har spiserørskreft, sammen med andre kreftformer med dårlig prognose, hatt en klar økning i overlevelse de siste 10–15 årene.
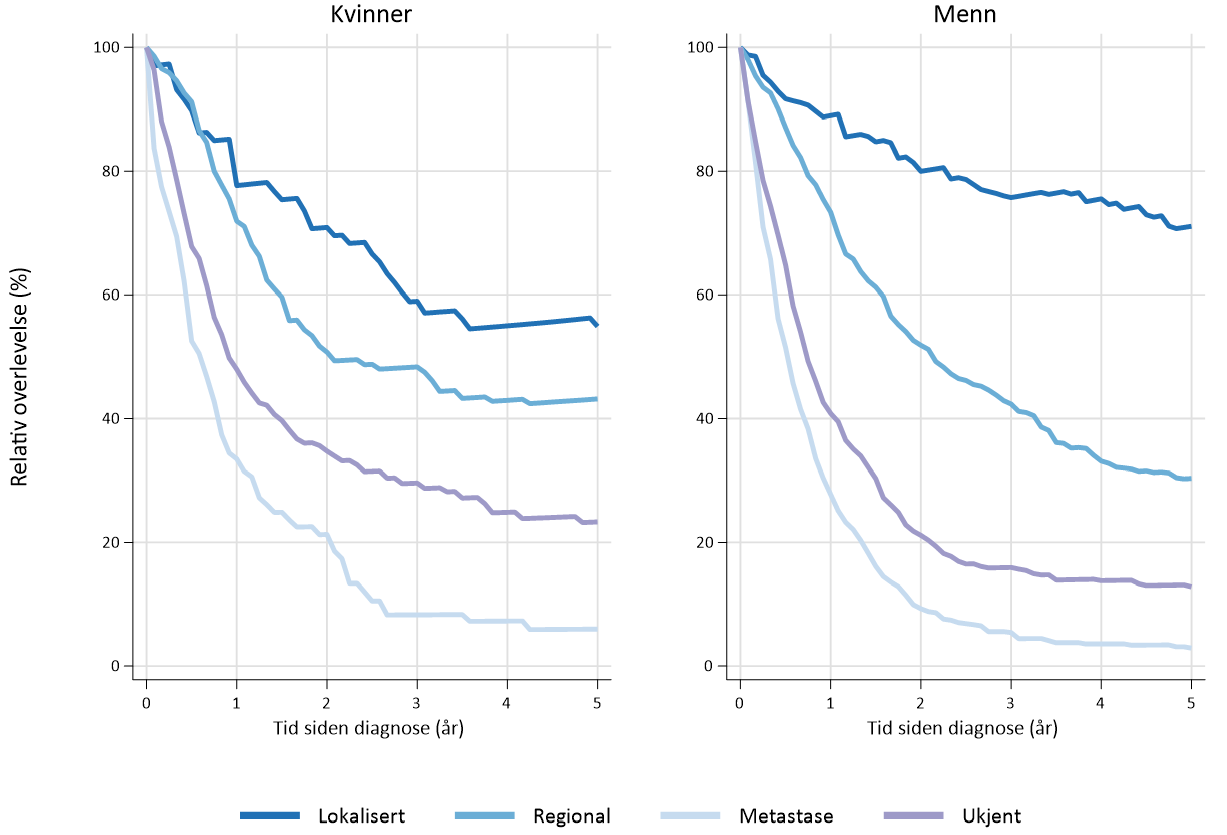
I perioden 2016–20 var estimert 5-års relativ overlevelse1 for menn 71 % ved lokalisert sykdom, 30 % ved regional sykdom og 3 % ved fjernmetastaser. Tilsvarende tall for kvinner i samme periode var 55 % ved lokalisert sykdom, 43 % ved regional sykdom og 6 % ved fjernmetastaser (Figur 1.2).
Fem-års overlevelse ved antatt kurativ reseksjon i den vestlige verden rapporteres fra 25–59 % (Hauge, Amdal, Falk, Johannessen, & Johnson, 2020; Hulscher & van Lanschot, 2005; Hulscher et al., 2002; Nuytens et al., 2021; Omloo et al., 2007; van Hagen et al., 2012). Stadiespesifikk relativ 5-års overlevelse etter reseksjon for spiserørskreft (platepitelkarsinom og adenokarsinom) basert på store internasjonale pasientmaterialer er 70–75 % for stadium IA, 60–65 % for IB, 50 % for IIA, 40–45 % for IIB, 25 % for IIIA, 17 % for IIIB og 15 % for IIIC.
1 Overlevelsesestimatene er basert på tall fra Kvalitetsregisteret, og avviker derfor fra overlevelsestall i Cancer in Norway 2020.
Forebygging og risikofaktorer
Sist faglig oppdatert: 28.06.2022
Spiserørskreft er klart relatert til livsstil, med tobakk og alkohol som de viktigste risikofaktorer for plateepitelkarsinom. Reflukssykdom er assosiert med økt risiko for adenokarsinom i spiserøret. Det samme er overvekt og fedme. Det finnes også andre faktorer som påvirker risikoen, og forebygging av nye tilfeller av spiserørskreft handler først og fremst om å unngå risikofaktorer, behandle forstadier til kreftsykdom, men også spesifikke intervensjoner synes å ha betydning.
Risikofaktorer
Sist faglig oppdatert: 28.06.2022
Plateepitelkarsinom
Selv mindre mengder alkohol (≤ 12.5 g/d) øker relativ risiko (RR: 1.38; 95 % CI: 1.14–1.67) for spiserørskreft. Ved moderat alkoholkonsum (>12.5 – 49 g/d) øker RR til 2.62 (95 % CI: 2.07–3.31) og ved høyt alkoholkonsum (≥50 g/d) er RR 5.54 (95 % CI: 3.92–7.28). Det foreligger interaksjon med røyking. Hos ikke-røykere innebærer lettere alkoholkonsum RR 0.74 (95 % CI: 0.47–1.16), moderat alkoholkonsum RR 1.54 (95 % CI: 1.09–2.17) og høyt alkoholkonsum RR 3.09 (1.75–5.46). Sammenhengen mellom alkohol og plateepitelkarsinom i spiserøret er mer uttalt i Asia enn i andre populasjoner (Islami et al., 2011).
Vedvarende røyking er forbundet med risiko for kreft både i munnhule og hals (Wyss et al., 2013) men også spiserør. Sammenlignet med personer som aldri har røyket innebærer aktiv røyking en assosiert risiko for plateepitelkarsinom i spiserøret (OR 4.17; 95 % CI: 2.45–7.10) (Lee et al., 2009). Det er vist at røyking har en sterkere assosiasjon til platecellekarisinomer enn andenokarsinomer i spiserør (World Cancer Research Fund/American Institute for Cancer Research, 2018b).
Ukentlige reflukssymptomer øker også risiko for plateepitelkarsinom (OR 2.2; 95 % CI: 1.5–3.2) (Pandeya, Webb, Sadeghi, Green, & Whiteman, 2010). Det er begrenset evidens for at bearbeidet kjøtt øker risikoen for plateepitelkarsinom i spiserør (World Cancer Research Fund/American Institute for Cancer Research, 2018b). I en nederlandsk studie ble det vist at bearbeidet og rødt kjøtt er assosiert med plateepitelkarsinom hos menn, men ikke hos kvinner. Hazard ratio for høyeste mot laveste kvintil er her 3.47 (95 % CI: 1.21–9.94). Tilsvarende forhold kunne ikke vises for adenokarsinom (Keszei, Schouten, Goldbohm, & van den Brandt, 2012).
Inntak av drikke med høy temperatur disponerer for termiske skader og kreft utgående fra plateepitel i spiserøret og bør unngås (Islami et al., 2009).
Humant papillomavirus (HPV) er hyppig til stede i kreft i munnhule (Powell, Boyde, Tristram, Hibbitts, & Fiander, 2009), men tilsvarende hyppighet ved plateepitelkarsinom i spiserøret er omdiskutert (Far et al., 2007).
Reevaluering av case-control studier viser at det er noe sammenheng mellom HPV infeksjon og plateepitelkarsinom i spiserøret OR 3.32; 95 % CI: 2.26–4.87) men sammenhengen er ikke så tydelig som ved kreft i livmorhals og hode hals. I en studie var prevalens av HPV ved plateepitelkarsinom i spiserøret 22.4 prosent og spesifikt var prevalens av HPV – 16, 11,4 % (Li et al., 2014). I en gjennomgang av 13 832 pasienter med plateepitelkarsinom i 124 studier varierte prevalens med region og anvendt deteksjons metode (Petrick et al., 2014). Prevalens av HPV var 26.4 % (95 % CI: 10.8, 42 %) med «Southern blot» og 41.6 % (33.1 %, 50.2 %) ved PCR teknikk i noen kinesiske regioner. Gjennomsnittlig prevalens av HPV i metaanalysen på tvers av regioner var 0.277 (0.234, 0.320) ved PCR; 0.243 (0.159, 0.326) ved in situ hybridisering; 0.304 (0.185 0.423) ved immunhistokjemi; 0.322 (0.154 0.490) ved serologi og 0.176 (0.061, 0.292) med «Southern blot». Funnene er ikke justert for prevalens av HPV i bakgrunnsbefolkningen. Høy prevalens av HPV i plateepitelkarsinom fra Kina og Afrika kan derfor ikke alene forklare geografiske variasjoner i insidens av plateepitelkarsinom i spiserøret.
Adenokarsinom
Reflukssykdom er assosiert med adenokarsinom i spiserøret (OR 7.77; 95 % CI: 5.3–11.4) og gastroøsofageal overgang (OR 2.0; 95 % CI: 1.4–2.9). Risikoen øker med varighet av refluks (>20 år) og intensitet av reflukssymptomene mot henholdsvis OR: 43.5 (95 % CI: 18.3–103.5) og 4.4 (95 % CI: 1.7–11.0) (Lagergren, Bergstrom, Lindgren, et al., 1999).
Målt ut fra ukentlige reflukssymptomer, øker risiko for adenkarsinom i spiserøret med OR 4.92 (95 % CI: 3.90–6.22) mens daglige symptomer er forbundet med OR 7.40 (95 % CI: 4.94–11.1) når man sammenligner personer uten eller med færre symptomer (Pandeya et al., 2010; Rubenstein & Taylor, 2010).
Tilsvarende er det påvist at daglig medikamentell påvirkning, utover 5 år med preparat som relakserer spiserørets nedre lukkemuskel (LES), innebærer risiko for utvikling av spiserørskreft (Insidens rate ratio 3.8; 95 % CI: 2.2–6.4). Risikoen var særlig høy for antikolinergika. Sammenhengen her synes å være mediert av reflukssykdom (Lagergren, Bergstrom, Adami, & Nyren, 2000).
Aktiv røyking er assosiert med kjønnsnøytral øket risiko for adenokarsinom og særlig i Barretts slimhinne (OR 2.08; 95 % CI: 1.83–2.37). Røyking innebærer også en dose respons assosiasjon med kreft mens opphør av røyking bidrar til å redusere risko for kreftsykdom (Cook et al., 2010; Tramacere, La Vecchia, & Negri, 2011) over tid. Kombinasjon av hyppig reflukssykdom og storrøyking innebærer en markert øket risiko for adenokarsinom i spiserøret (OR 12.3; 95 % CI: 6.3–24.0) (Pandeya et al., 2010).
Selv om reflukssykdom innebærer risiko for kreft i spiserøret er det ikke dokumentert at antireflukskirurgi reduserer risikoen de første 15 år etter operasjonen (Lagergren, Ye, Lagergren, & Lu, 2010; Löfdahl, Lu, Lagergren, & Lagergren, 2013; Maret-Ouda, Konings, Lagergren, & Brusselaers, 2016).
Overvekt er assosiert med en kjønnsnøytral risiko for adenokarsinom i spiserør og gastroøsofageal overgangen. Ved KMI 25–30 kg/m2 er relativ risiko: 1.71 (95 % CI: 1.5–1.96) og for KMI ≥30 kg/m2er relativ risiko 2.34 (95 % CI: 1.95–2.81) (Turati, Tramacere, La Vecchia, & Negri, 2013).
Helicobacter pylori (Hp) er ikke assosiert med adenokarsinom i spiserør. Faktisk foreligger et inverst forhold mellom tilstedeværelse av Hp og adenokarsinom (OR 0.52; 95 % CI: 0.37–0.73) og Barrett’s slimhinne (OR 0.64; 95 % CI: 0.43–0.94) (Rokkas, Pistiolas, Sechopoulos, Robotis, & Margantinis, 2007) i spiserøret.
Det er ikke identifisert sikker sammenheng mellom alkoholinntak (Tramacere et al., 2012) og adenokarsinom i spiserøret.
Forebygging
Sist faglig oppdatert: 28.06.2022
Plateepitelkarsinom
En meta-analyse av observasjonsstudier har vist at høyt inntak av grønnsaker RR 0.56 (95 % CI: 0.45–0.69) og frukt 0.53 (95 % CI: 0.44–0.64) reduserer risiko for utvikling av plateepitelkarsinom i spiserøret (Liu, Wang, Leng, & Lv, 2013). Likevel konkluderer rapporten fra WCRF/AICR med at det finnes begrensede bevis for en beskyttende effekt av både grønnsaker og frukt (World Cancer Research Fund/American Institute for Cancer Research, 2018b).
NSAIDs er i metaanalyser på observasjonsstudier vist å redusere risiko for kreftutvikling (OR 0.58; 95 % CI: 0.47–0.72) (L. Sun & Yu, 2011).
Adenokarsinom
En antar at det for adenokarsinom er forebyggende å unngå overvekt og å behandle eventuelle refluksplager. En har ikke kunnet dokumentere at antirefluksmedikasjon eller antireflukskirurgi reduserer forekomsten av adenokarsinom i spiserøret. Regelmessig inntak av ASA/NSAIDS hos personer med mye og/eller hyppig refluks reduserer risiko for adenokarsinom i spiserøret til OR 4.8; 95 % CI: 2.5–9.2) (Pandeya et al., 2010) når det sammenlignes med de umedisinerte (OR 13.9; 95 % CI: 6.5–30.0). Syrehemmer ved reflukssykdom beskyttet ikke mot kreftutvikling (Pandeya et al., 2010).
Metaanalyser av observasjonsstudier viser at også statiner reduserer risiko for kreft i spiserøret særlig hos pasienter med Barretts slimhinne (OR 0.59; 95 % CI: 0.45–0.78) (Singh, Singh, Singh, Murad, & Iyer, 2013). NSAID (HR 0.48; 95 % CI: 0.23–1.01) og statiner (HR 0.48; 95 % CI: 0.19–1.21) reduserte risiko for progresjon til høygradig dysplasi eller kreft i Barretts slimhinne, mens kombinasjonsbehandling potenserer effekten ytterligere (HR 0.24; 95 % CI: 0.07–0.82) (Kastelein et al., 2011).
Resultater av kjemoprevensjon i case–control-studier må likevel reproduseres i randomiserte kliniske studier.
Forløpstider
Sist faglig oppdatert: 28.06.2022
Fra 1. mai 2015 ble Pakkeforløp for kreft i spiserør og magesekk innført. Da ble tidligere forløpstider erstattet av de nye tidene i Pakkeforløp for kreft i spiserør og magesekk.
Om Pakkeforløp for kreft
Sist faglig oppdatert: 28.06.2022
Pakkeforløp for kreft skal gi forutsigbarhet og trygghet for pasient og pårørende, og er et standard pasientforløp som beskriver organisering av utredning og behandling, kommunikasjon/dialog med pasient og pårørende samt ansvarsplassering og konkrete forløpstider. Pakkeforløpet starter når et helseforetak eller privat ideelt sykehus mottar en henvisning med begrunnet mistanke om kreft, eller når helseforetaket selv starter utredning med begrunnet mistanke om kreft.
Formålet med Pakkeforløp for kreft er at kreftpasienter skal oppleve et godt organisert, helhetlig og forutsigbart forløp uten unødvendig ikke-medisinsk begrunnet forsinkelse i utredning, diagnostikk, behandling og rehabilitering.
Forløpstidene i pakkeforløpet beskriver den maksimale tiden de ulike fasene i forløpet bør ta. Forløpstidene angis i kalenderdager. De enkelte fasenes forløpstid legges til slutt sammen til en samlet forløpstid, som angir tiden fra henvisning er mottatt til behandling er startet. Med utgangspunkt i pakkeforløpet skal et individuelt forløp tilrettelegges for hver enkelt pasient.
De regionale helseforetakene har det overordnede ansvaret for å sikre at pakkeforløpene med forløpstidene blir implementert og fulgt opp. Forløpstidene er normerende og er ikke en pasientrettighet. Fortsatt er det lovmessige grunnlaget pasientrettighetsloven § 2-2 og forskrift om prioritering av helsetjenester. Av og til vil det av faglige grunner være noen pasienter som ikke kan utredes ferdig innen normert forløpstid for oppstart av første behandling. Årsaker til avvik fra de normerte forløpstidene bør dokumenters i pasientjournalen.
Forløpstider for spiserørskreft
Sist faglig oppdatert: 28.06.2022
I Pakkeforløp for kreft i spiserør og magesekk er det utarbeidet følgende forløpstider
| Fra henvisning mottatt til første fremmøte i utredende avdeling |
| 8 kalenderdager |
| Fra første fremmøte i utredende avdeling til avsluttet utredning (beslutning tas) |
| 21 kalenderdager |
| Fra avsluttet utredning til start behandling | Kirurgisk behandling | 14 kalenderdager |
| Fra avsluttet utredning til start behandling | Medikamentell behandling | 14 kalenderdager |
| Fra avsluttet utredning til start behandling | Strålebehandling | 14 kalenderdager |
| Fra henvisning mottatt til start behandling | Kirurgisk behandling | 43 kalenderdager |
| Fra henvisning mottatt til start behandling | Medikamentell behandling | 43 kalenderdager |
| Fra henvisning mottatt til start behandling | Strålebehandling | 43 kalenderdager |
Pakkeforløp for kreft i spiserør og magesekk finnes på Helsedirektoratets nettsider.
Det er utarbeidet egne diagnoseveiledere for fastleger for inngang til pakkeforløp. Diagnoseveileder for kreft i spiserør og magesekk kreft finnes på Helsedirektoratets nettsider.
Diagnose og utredning
Sist faglig oppdatert: 28.06.2022
Selve utvelgelsen av pasienter med spiserørskreft til adekvat behandling er avhengig av nøyaktig stadieinndeling av kreftsykdommen. I review-undersøkelser har man funnet at resultatene med henblikk på stadieinndeling, beslutning vedrørende behandling, komplikasjoner og perioperativ mortalitet er bedret når pasientene blir behandlet av multidisiplinære team (MDT) ved høy-volum sentra med spesiell kompetanse (Allum et al., 2011; Davies et al., 2006; Halm, Lee, & Chassin, 2002; Killeen, O'Sullivan, Coffey, Kirwan, & Redmond, 2005). I MDT for vurdering av spiserørskreft inngår radiolog, kirurg og onkolog (Allum et al., 2011). Behandlingen bør derfor sentraliseres.
Anbefaling
Pasienter med mulig operabel spiserørskreft bør behandles ved sentra med spesiell kompetanse og evalueres av multidisiplinære team (evidensgrad C).
Symptomer
Sist faglig oppdatert: 28.06.2022
Debutsymptom er hos de aller fleste pasienter relatert til primærtumor, og dette kan være svelgvansker, slimdannelse og vekttap. Mange erfarer at kjøttbiter setter seg midlertidig fast, og at dette tiltar i hyppighet. Smerter, heshet (innvekst i nervus recurrens), hoste og aspirasjonspneumoni (gjennomvekst til trachea/bronkier) er sene symptomer. Ved gastroøsofageal refluks som årsak til kreft er det kun omtrent halvparten av pasientene som tidligere har oppsøkt lege for refluksplager, og mange kommer til sin første gastroskopi i forbindelse med kreftdiagnosen. Pasienter med reflukssymptomer har gjerne hatt dette i en årrekke.
Utredning
Sist faglig oppdatert: 28.06.2022
Endoskopi: Pasienter som søker lege for nyoppstått dysfagi bør som første ledd i utredning henvises til gastroskopi.
Ved begrunnet mistanke om kreft, henvises pasienten til Pakkeforløp for kreft i spiserør og magesekk. Halvparten av pasientene har ikke tidligere vært til gastroskopi, mens et fåtall har gått til årelange kontroller for øsofagitt og celleforandringer i spiserøret (Barrettforandringer med dysplasi).
Synlig tumor skal biopseres (tilstrekkelig antall og dype nok biopsier), og i skopibeskrivelsen skal avstand fra tannrekken til tumors proksimale og distale utbredelse angis, dessuten beliggenhet i forhold til z-linjen og toppen av ventrikkelfoldene. I tillegg skal det angis hvor stor del av omkretsen tumor inntar. Det samme gjelder ved flere separate svulster. Ikke sjelden finnes submukosal vekst, noe som kan gi seg til kjenne ved multiplisitet endoskopisk, og som diskrepans i tumorlengde mellom skopi- og CT-funn.
For å oppdage minimale og tidlige celleforandringer har man de senere år utviklet en rekke former for spesialisert endoskopi: kromoendoskopi, høyoppløsningsendoskopi, visuell kromoendoskopi og konfokal endoskopi. Det har vært vanskelig å påvise en nytteeffekt av de ulike metodene. Derfor er det ikke aktuelt å innføre disse som rutinemetode (Mannath, Subramanian, Hawkey, & Ragunath, 2010). Ved Barretts øsofagus og kontroll av dysplasi er det av stor betydning å følge en strukturert protokoll for biopsitaking. Det skal ved første undersøkelse tas biopsier av alle fire kvadranter med to cm avstand i hele utstrekningen av Barrettforandringene (Fitzgerald et al., 2014; Mannath et al., 2010; Phoa et al., 2014; Ragunath, Krasner, Raman, Haqqani, & Cheung, 2003).
Anamnese/funn: Aktuell vekt, vekttap siste seks måneder og funksjonsgrad (ECOG/WHO) skal registreres i pasientjournalen da det har betydning for prognose, valg av behandling og behov for ernæringstiltak.
| ECOG/WHO funksjonsgrad 0. Asymptomatisk. |
Vurdering ved øre-nese-hals-lege kan være indisert ved svulster i øvre del av spiserøret.
EUS: Er best for lokoregional stadiuminndeling men har en del begrensninger. FNA hever resultatene (Choi, Kim, Kim, Jung, & Song, 2010; Khanna & Gress, 2015; Winiker et al., 2018).
CT: Undersøkelse av hals, thorax og øvre abdomen, der dedisert spesialprosedyre (hydro-CT) har høyest sensitivitet og diagnostisk nøyaktighet. Undersøkelsen taes som ett sammenhengende volum fra kjevevinkel til nedre leverspiss, med distendert spiserør i to kontrastfaser. Da får man god framstilling av T3- og T4-tumores, ofte også av T2-tumores – med utbredelse i lengde og dybde, affeksjon av nabostrukturer samt metastaser til lever og lunger (Ba-Ssalamah et al., 2011; Ringe et al., 2015; Ulla et al., 2012). Samtidig avbilding av bekkenet er nyttig for å kunne avdekke carcinomatose. CT har lavere spesifisitet for deteksjon av spredning til mediastinale lymfeknuter, spesielt de tumornære, bedre for lymfeknuter i cøliakusgebetet. Undersøkelsen gir best anatomisk kartlegging for planlegging av kirurgisk inngrep. Prosedyren bør utføres ved avdeling med spesiell radiologisk kompetanse og helst ved den institusjon hvor pasienten skal behandles.
PET: PET har ingen rolle ved vurdering av tumors T-stadium (Bruzzi et al., 2007; Dehdashti & Siegel, 2006). PET synes også å gi lite tilleggsinformasjon om regional lymfeknutestatus (CT = PET) (Bruzzi et al., 2007; Dehdashti & Siegel, 2006; Khanna & Gress, 2015). PET har høyere sensitivitet enn CT for påvisning av okkulte fjernmetastaser og anbefales ved plateepitelkarsinom og T3N+ adenokarsinomer der dette får terapeutiske konsekvenser. Det foreligger heller ikke god dokumentasjon av nytteverdi for vurdering av behandlingsrespons (van Rossum et al., 2015). PET er aktuelt ved spørsmål om residiv der annen bildediagnostikk er inkonklusiv og det får terapeutiske konsekvenser (van Rossum et al., 2015). Det er foreløpig ikke dokumentasjon for at det er nødvendig å utføre PET på alle pasienter med kreft i spiserøret.
MR: Det er foreløpig ingen dokumentasjon for at MR gir mer korrekt stadiuminndeling enn CT og EUS. MR er indisert hvis det foreligger usikkerhet ved vurdering av mulig malignitet ved påviste leverlesjoner (MR bedre enn PET-CT for påvisning av små levermetastaser) eller skjelettforandringer.
Kombinasjon ev EUS-FNA og CT gir best lokoregional kartlegging.
Røntgen spiserøret/magesekk har ingen rutinemessig plass i primærutredningen, men kan eventuelt gjøres som tilleggsundersøkelse for å utrede stenoser
Anbefalinger
- Pasienter med nyoppstått dysfagi skal henvises til øvre endoskopi (evidensgrad D).
- Ved påvist spiserørskreft tas CT hals, thorax og abdomen samt bekken, som dedisert spesialprosedyre (evidensgrad B).
- EUS anbefales for stadieinndeling når dette kan få terapeutiske konsekvenser (evidensgrad A).
- MR er ikke indisert rutinemessig (evidensgrad A).
- PET-CT anbefales ved plateepitelkarsinom og T3N+ adenokarsinomer for deteksjon av fjernmetastaser når dette kan få terapeutiske konsekvenser (evidensgrad B)
Patologi – Biopsibesvarelse
Sist faglig oppdatert: 28.06.2022
Generelt
En biopsibesvarelse skal, så langt det er mulig, svare på følgende:
- Foreligger det et infiltrerende karsinom eller bare dysplasi (intraepitelial neoplasi)
- Dersom det foreligger dysplasi, er denne lavgradig eller høygradig
- Dersom det foreligger et infiltrerende karsinom, er dette plateepitelkarsinom, adenokarsinom eller evt. annen tumor
Dersom det morfologisk er vanskelig å avgjøre hva slags tumor som foreligger, bør det gjøres immunhistokjemisk undersøkelse (eks. CK5/6 og p40 for å påvise plateepiteldifferensiering).
Platepitelkarsinom
Plateepitelkarsinomer i spiserøret utvikles via lavgradig og høygradig dysplasi (intraepitelial neoplasi) (International Agency for Research on Cancer, 2019).
Adenokarsinom
De fleste adenokarsinomer i spiserøret utvikles fra Barretts øsofagus (intestinal metaplasi), via lavgradig og høygradig dysplasi (intraepitelial neoplasi) (International Agency for Research on Cancer, 2019). Begrepet uviss dysplasi kan benyttes ved lett cellulær og/eller arkitektonisk irregularitet som ikke tilfredsstiller kravene til dysplasi, ved betennelse og ulcerasjon, dersom biopsimaterialet ikke er optimalt og dersom de cellulære forandringer ikke når overflateepitelet.
Ved Barretts øsofagus er muskularis mukosa ofte fortykket og oppsplittet. Dersom tumor infiltrerer i, men ikke gjennom muskularis mukosa, oppfattes dette som et intramukosalt karsinom.
Adenokarsinomer i spiserøret skal alltid undersøkes mhp. HER-2, primært ved immunhistokjemisk undersøkelse (Hofmann et al., 2008).
| Immunhistokjemisk | HER-2 (Bosman, Carneiro, Hruban, & Theise, 2010) |
| 0 – +1 | Negativ |
| +2 | Usikker, gjør in situ hybridisering (FISH eller SISH) |
| +3 | Positiv |
Etter forespørsel fra kliniker kan tumor undersøkes mhp. MSI/MMR, EBV (in situ hybridisering) og PD-L1 (immunhistokjemi). Ved PD-L1 testing av karsinomer i øsofagus benyttes «Combined Positive Score» (CPS) (Agilent Technologies, 2019) dersom det foreligger adenokarsinom eller plateepitelkarsinom. Ved plateepitelkarsinom benyttes i tillegg «Tumor Proportion Score» (TPS).
Disse beregnes på følgende måte:

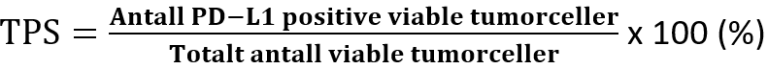
Selv om resultatet av utregningen av CPS kan overskride 100, er maksimal score definert som CPS 100. Vedrørende rapportering med TPS, finnes på nåværende tidspunkt ingen kjente retningslinjer for hvordan denne skal beregnes på øsofagealt PEK, og inntil videre beregnes og rapporteres denne på samme måte som for lungekarsinomer (Agilent Technologies, 2018)
Stadieinndeling
Sist faglig oppdatert: 28.06.2022
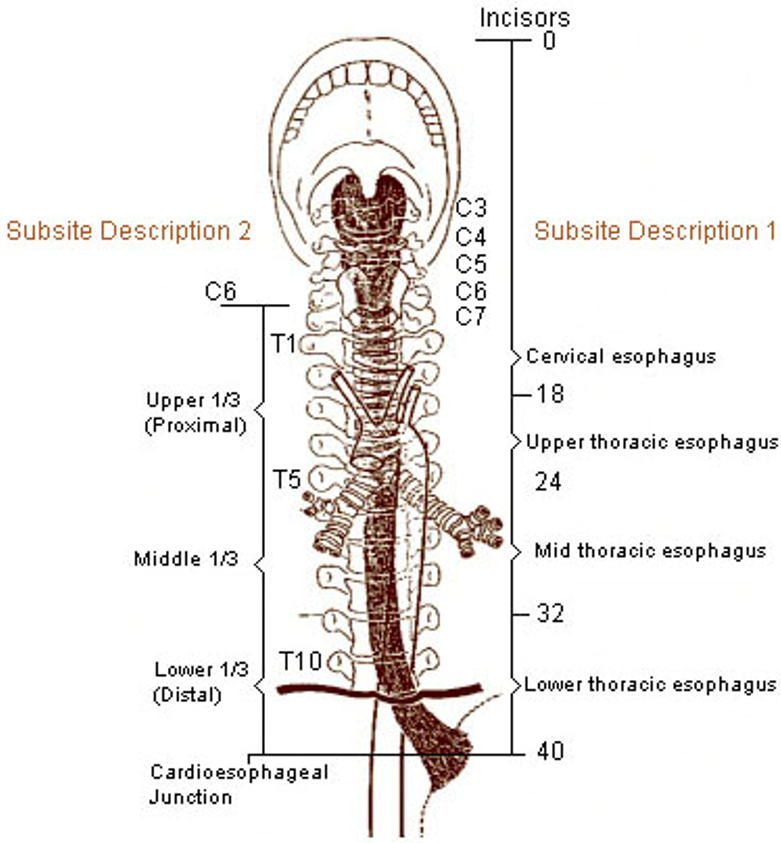
Klassifikasjon og stadieinndeling skal utføres i henhold til UICC/TNM klassifikasjon 8. utgave, 2017 (Brierley, Gospodarwicz, & Wittekind, 2017). Svulster med episenter ≤ 2 cm distalt for gastroøsofageale overgang (øvre begrensning av ventrikkelfoldene) og som også strekker seg proksimalt for denne, stadieinndeles og klassifiseres som spiserørskreft.
- Cervicale øsofagus (C15.0): fra nedre kant av cricoidbrusken til jugulum (halsgropen), ca. 18 cm fra øvre tannrekke.
- Intratorakale øsofagus
- Øvre torakale (C15.3) fra jugulum til trakealbifurkaturen, ca. 24 cm fra øvre tannrekke
- Midtre torakale (C15.4) er proksimale halvdel av øsofagus fra trakealbifurkaturen til gastroøsofageale overgang. Distale avgrensning ca. 32 cm fra øvre tannrekke.
- Nedre torakale (C15.5) ca. 8 cm lang (inkluderer abdominale øsofagus). Distale halvdel av øsofagus mellom trakealbifurkaturen og gastroøsofageale overgang. Distale ende ca. 40 cm frå øvre tannrekke.
- Gastroøsofageale overgang (C16.0).
Regionale lymfeknuter, uavhengig av primærtumors lokalisasjon, er lymfeknuter i drenasjeområdet for spiserøret, inklusive lymfeknuter ved truncus coeliacus og paraøsofageale lymfeknuter på halsen, men ikke supraclaviculære lymfeknuter.
TNM-klassifikasjon
TX = Primærtumor kan ikke vurderes
T0 = Ikke påvist primærtumor
Tis = Carcinoma in situ/høygradig dysplasi
T1 = Infiltrasjon i lamina propria, muscularis mucosae eller submucosa
T1a = Infiltrasjon i lamina propria, muscularis mucosae
T1b = Infiltrasjon i submucosa
T2 = Infiltrasjon i muscularis propria
T3 = Infiltrasjon i adventitia, men ikke inn i andre organer/strukturer
T4 = Infiltrasjon i andre organer eller strukturer
T4a = Tumor som infiltrerer plevra, perikard, vena azygos eller diafragma
T4b = Tumor som infiltrerer nabostrukturer som f.eks. aorta, hvirvelcorpus, trakea
NX = Regionale lymfeknuter kan ikke vurderes
N0 = Ingen regionale lymfeknutemetastaser
N1 = Spredning til 1-2 regionale lymfeknuter
N2 = Spredning til 3-6 regionale lymfeknuter
N3 = Spredning til 7 eller flere regionale lymfeknuter
M0 = Ingen fjernmetastaser
M1 = Fjernmetastaser
Klinisk TNM-stadium
Sist faglig oppdatert: 28.06.2022
Plateepitelkarsinom
| STADIUM | T | N | M |
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| I | T1 | N0, N1 | M0 |
| II | T2 | N0, N1 | M0 |
| T3 | N0 | M0 | |
| III | T3 | N1 | M0 |
| Any T | N2 | M0 | |
| IVA | T4a, T4b | N0, N1, N2 | M0 |
| Any T | N3 | M0 | |
| IVB | Any T | Any N | M1 |
Adenokarsinom
| STADIUM | T | N | M |
|---|---|---|---|
| 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| IIA | T1 | N1 | M0 |
| IIB | T2 | N0 | M0 |
| III | T2 | N1 | M0 |
| T3, T4a | N0, N1 | M0 | |
| IVA | T1-T4a | N2 | M0 |
| T4b | N0, N1, N2 | M0 | |
| Any T | N3 | M0 | |
| IVB | Any T | Any N | M1 |
Patologisk TNM-stadium
Plateepitelkarsinom
| STADIUM | T | N | M | GRAD | Tumorlokalisasjon |
|---|---|---|---|---|---|
| 0 | Tis (HGD) | N0 | M0 | 1, X | Any |
| IA | T1 | N0 | M0 | 1, X | Any |
| IB | T1 | N0 | M0 | 2–3 | Any |
| T2–3 | N0 | M0 | 1, X | Lower, X | |
| IIA | T2–3 | N0 | M0 | 1, X | Upper, middle |
| T2–3 | N0 | M0 | 2–3 | Lower, X | |
| IIB | T2–3 | N0 | M0 | 2–3 | Upper, middle |
| T1–2 | N1 | M0 | Any | Any | |
| IIIA | T1–2 | N2 | M0 | Any | Any |
| T3 | N1 | M0 | Any | Any | |
| T4a | N0 | M0 | Any | Any | |
| IIIB | T3 | N2 | M0 | Any | Any |
| IIIC | T4a | N1–2 | M0 | Any | Any |
| T4b | Any | M0 | Any | Any | |
| Any | N3 | M0 | Any | Any | |
| IV | Any | Any | M1 | Any | Any |
Adenokarsinom
| STADIUM | T | N | M | GRAD |
|---|---|---|---|---|
| 0 | Tis (HGD) | N0 | M0 | 1, X |
| IA | T1 | N0 | M0 | 1–2, X |
| IB | T1 | N0 | M0 | 3 |
| T2 | N0 | M0 | 1–2, X | |
| IIA | T2 | N0 | M0 | 3 |
| IIB | T3 | N0 | M0 | Any |
| T1–2 | N1 | M0 | Any | |
| IIIA | T1–2 | N2 | M0 | Any |
| T3 | N1 | M0 | Any | |
| T4a | N0 | M0 | Any | |
| 0IIIB | T3 | N2 | M0 | Any |
| IIIC | T4a | N1–2 | M0 | Any |
| T4b | Any | M0 | Any | |
| Any | N3 | M0 | Any | |
| IV | Any | Any | M1 | Any |
Behandling av dysplasi og kreft i tidlig stadium
Barretts øsofagus med lavgradig dysplasi
Sist faglig oppdatert: 28.06.2022
Barretts øsofagus (BØ) er en premalign tilstand som disponerer for høygradig dysplasi (Tis, HGD) og adenokarsinom (AK) i spiserøret, antall pasienter med kreft er økende. For å stille diagnosen må forandringene ved gastroskopi være av minst 1 cm lengde og inneholde den typiske intestinale metaplasien ved histopatologisk undersøkelse.
Ved gastroskopi må CM-klassifikasjonen anvendes (Praha kriteriene), der C er sirkulær og M maksimal utbredelse. Funnet må dokumenteres med et bilde der også lokaliserte forandringer innenfor BØ dokumenteres og angis i cm fra tannrekken.
Det skal tas 4-kvadrant biopsier hver 2. cm innenfor BØ på nummererte glass.
Ved fravær av dysplasi skal BØ med lengde 1–3 cm kontrolleres etter 5 år, 3–10 cm etter 3 år. Om BØ lengde er ≥10 cm skal pasienten henvises til et ekspertsenter, i praksis regionsykehus som gjør spiserørskirurgi. Etter fylte 75 år uten forekomst av dysplasi, er det ikke nødvendig med fortsatt endoskopisk overvåking. Ved uviss (indefinite) dysplasi tas kontroll biopsier etter 6 mndr med optimalisert refluksbehandling (PPI).
Alle synlige forandringer innenfor BØ skal reseseres for å oppnå optimal histopatologisk staging. Funn av lavgradig dysplasi (LGD) bekreftet av en en erfaren GI-patolog, anbefales kontrollert etter seks måneder. Om dysplasi da ikke kan bekreftes, anbefales en ny kontroll etter ett år, og etter to negative kontroller standard overvåking, dvs avhengig av BØs lengde. Om derimot LGD bekreftes, skal pasienten tilbys radiofrekvensablasjon (RFA) (di Pietro, Fitzgerald, & BSG Barrett's guidelines working group, 2018; Phoa et al., 2014; Weusten et al., 2017).
Barretts øsofagus med høygradig dysplasi og/eller adenokarsinom i tidlig stadium
Sist faglig oppdatert: 28.06.2022
I tillegg til behandling av LGD, er følgende også i tråd med de siste ESGE retningslinjene som kom i 2017 (Weusten et al., 2017). Disse beskriver dessuten nødvendige krav til kompetanse og erfaring som et ekspertsenter skal inneha, inkludert en prospektiv registrering av pasienter med BØ i en database.
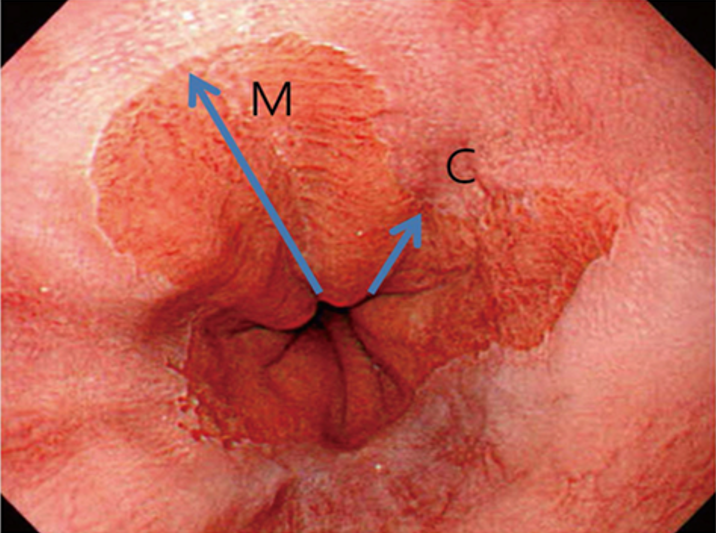
Ved bekreftet høygradig dysplasi (HGD, Tis) –og/eller adenokarsinom, T1a, skal pasienten tilbys endoskopisk behandling. Dette innebærer reseksjon av lokaliserte forandringer (EMR) kombinert med RFA av resterende BØ. Endoskopisk submukosal disseksjon (ESD) har ikke vist seg å gi bedre resultater enn EMR, som er en enklere og tidsmessig raskere teknikk (Komeda, Bruno, & Koch, 2014; Terheggen et al., 2017). «Multiband mucosectomy» eller gummibånd EMR er den enkleste og foretrukne metode. Ved T1b kan EMR også være aktuelt om pasienten er «borderline fit» for kirurgi, forutsatt fravær av lymfeknuter og en begrenset submukosal innvekst (<500 µm). Dette er pasienter som må diskuteres nøye i MDT møter for spiserørskreft.
Høygradig dysplasi uten synlige lesjoner er sjelden, og utgjør færre enn 20% av pasientene med HGD. Fravær av synlige lesjoner hos en pasient med HGD, kan være en oversett lesjon, eller over-staging av histologien (66). Om pasienten ikke allerede er i et pakkeforløp, er start pakkeforløp aktuelt ved HGD.
Etter endoskopisk behandling bør kontroll med gastroskopi foretas hver 3. måned i ett år, deretter årlig. Ved eventuelt BØ bør hele området med BØ forsøkes fjernet med RFA.
For dysplasi og kreft I tidlig stadium utgående fra plateepitel (PE) gjelder det samme for endoskopisk behandling. Det betyr også her kirurgi fra og med T1b.
Ved lesjoner over 15 mm, manglende løft og usikkerhet om innvekst i submukosa, vil om ikke kirurgi er aktuelt, ESD i erfarende hender være bedre enn EMR (Malik, Sharma, Sanaka, & Thota, 2018).
%20i%20spiser%C3%B8ret.png)
Anbefalinger
Pasienter med dysplasi og intramukosalt karsinom bør få tilbud om endoskopisk behandling (evidensgrad C)
Endoskopisk behandling bør sentraliseres til få sykehus (evidensgrad D).
Barretts øsofagus med lavgradig dysplasi
Sist faglig oppdatert: 28.06.2022
Barretts øsofagus (BØ) er en premalign tilstand som disponerer for høygradig dysplasi (Tis, HGD) og adenokarsinom (AK) i spiserøret, antall pasienter med kreft er økende. For å stille diagnosen må forandringene ved gastroskopi være av minst 1 cm lengde og inneholde den typiske intestinale metaplasien ved histopatologisk undersøkelse.
Ved gastroskopi må CM-klassifikasjonen anvendes (Praha kriteriene), der C er sirkulær og M maksimal utbredelse. Funnet må dokumenteres med et bilde der også lokaliserte forandringer innenfor BØ dokumenteres og angis i cm fra tannrekken.
Det skal tas 4-kvadrant biopsier hver 2. cm innenfor BØ på nummererte glass.
Ved fravær av dysplasi skal BØ med lengde 1–3 cm kontrolleres etter 5 år, 3–10 cm etter 3 år. Om BØ lengde er ≥10 cm skal pasienten henvises til et ekspertsenter, i praksis regionsykehus som gjør spiserørskirurgi. Etter fylte 75 år uten forekomst av dysplasi, er det ikke nødvendig med fortsatt endoskopisk overvåking. Ved uviss (indefinite) dysplasi tas kontroll biopsier etter 6 mndr med optimalisert refluksbehandling (PPI).
Alle synlige forandringer innenfor BØ skal reseseres for å oppnå optimal histopatologisk staging. Funn av lavgradig dysplasi (LGD) bekreftet av en en erfaren GI-patolog, anbefales kontrollert etter seks måneder. Om dysplasi da ikke kan bekreftes, anbefales en ny kontroll etter ett år, og etter to negative kontroller standard overvåking, dvs avhengig av BØs lengde. Om derimot LGD bekreftes, skal pasienten tilbys radiofrekvensablasjon (RFA) (di Pietro, Fitzgerald, & BSG Barrett's guidelines working group, 2018; Phoa et al., 2014; Weusten et al., 2017).
Barretts øsofagus med høygradig dysplasi og/eller adenokarsinom i tidlig stadium
Sist faglig oppdatert: 28.06.2022
I tillegg til behandling av LGD, er følgende også i tråd med de siste ESGE retningslinjene som kom i 2017 (Weusten et al., 2017). Disse beskriver dessuten nødvendige krav til kompetanse og erfaring som et ekspertsenter skal inneha, inkludert en prospektiv registrering av pasienter med BØ i en database.
Ved bekreftet høygradig dysplasi (HGD, Tis) –og/eller adenokarsinom, T1a, skal pasienten tilbys endoskopisk behandling. Dette innebærer reseksjon av lokaliserte forandringer (EMR) kombinert med RFA av resterende BØ. Endoskopisk submukosal disseksjon (ESD) har ikke vist seg å gi bedre resultater enn EMR, som er en enklere og tidsmessig raskere teknikk (Komeda, Bruno, & Koch, 2014; Terheggen et al., 2017). «Multiband mucosectomy» eller gummibånd EMR er den enkleste og foretrukne metode. Ved T1b kan EMR også være aktuelt om pasienten er «borderline fit» for kirurgi, forutsatt fravær av lymfeknuter og en begrenset submukosal innvekst (<500 µm). Dette er pasienter som må diskuteres nøye i MDT møter for spiserørskreft.
Høygradig dysplasi uten synlige lesjoner er sjelden, og utgjør færre enn 20% av pasientene med HGD. Fravær av synlige lesjoner hos en pasient med HGD, kan være en oversett lesjon, eller over-staging av histologien (66). Om pasienten ikke allerede er i et pakkeforløp, er start pakkeforløp aktuelt ved HGD.
Etter endoskopisk behandling bør kontroll med gastroskopi foretas hver 3. måned i ett år, deretter årlig. Ved eventuelt BØ bør hele området med BØ forsøkes fjernet med RFA.
For dysplasi og kreft I tidlig stadium utgående fra plateepitel (PE) gjelder det samme for endoskopisk behandling. Det betyr også her kirurgi fra og med T1b.
Ved lesjoner over 15 mm, manglende løft og usikkerhet om innvekst i submukosa, vil om ikke kirurgi er aktuelt, ESD i erfarende hender være bedre enn EMR (Malik, Sharma, Sanaka, & Thota, 2018).

%20i%20spiser%C3%B8ret.png)

Anbefalinger
Pasienter med dysplasi og intramukosalt karsinom bør få tilbud om endoskopisk behandling (evidensgrad C)
Endoskopisk behandling bør sentraliseres til få sykehus (evidensgrad D).
Kirurgisk og onkologisk behandling med kurativ intensjon
Kirurgisk behandling
Sist faglig oppdatert: 28.06.2022
Kirurgisk behandling planlegges nærmest uten unntak i kurativ hensikt. Prognosen er først og fremst relatert til sykdomsstadium på operasjonstidspunktet og om det ved operasjonen oppnås en R0-reseksjon eller ikke. Totalt regner en med at omkring 20 % av pasientene med spiserørskreft gjennomgår operasjon med kurativ intensjon (Jamieson, Lamb, & Thompson, 2009). Spiserørskirurgi bør utføres der det er tilstrekkelig logistikk for god preoperativ vurdering og tilstrekkelig antall pasienter (Allum et al., 2011; Davies et al., 2006; Halm et al., 2002; Killeen et al., 2005).
Kriterier for kirurgi
For pasienter med spiserørskreft T1bN0M0 anbefales operasjon direkte. For pasienter med sykdom i mer avansert stadium anbefales neoadjuvant behandling i tillegg.
T4b svulster (innvekst i ikke-resektabelt naboorgan) og svulster med fjernmetastaser opereres vanligvis ikke. Dette betyr at pasienter med følgende funn primært ikke tilbys øsofagusreseksjon: Pasienter med fjernmetastaser, innvekst i lunge, trachea, aorta eller spredning til ikke–regionale lymfeknuter, dvs. supraklavikulært eller para-aortalt kaudalt for nyrearteriene. Kirurgisk behandling av såkalt oligometastatisk sykdom (noen få lunge eller levermetastaser) er omdsikutert og det foreligger ingen entydig definisjon av oligometastatisk sykdom (S. R. Markar et al., 2016). Kirurgisk behandling av pasienter med oligometastatisk sykdom må derfor drøftes på individuell basis på tverrfaglige møter og kan vurderes særlig hos unge pasienter med få metastaser som forsvinner under neoadjuvant behandling.
Pasienter med alvorlig komorbiditet (organsvikt), især alvorlig hjerte/lungesykdom, er vanligvis ikke kandidater for øsofagusreseksjon. Det samme gjelder pasienter med sterkt nedsatt funksjon og/eller samarbeidsevne.
Inndeling av kreft i overgangen mellom spiserør/magesekk
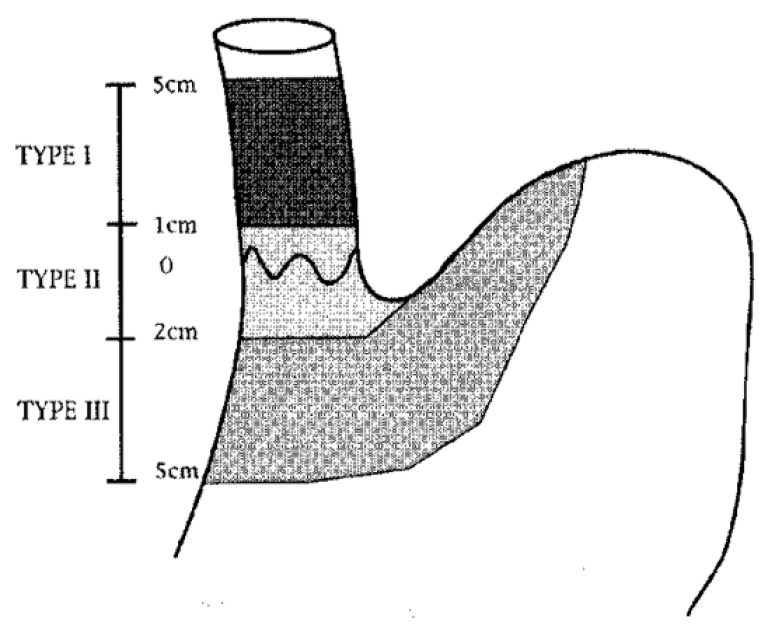
Siewerts klassifikasjon:
Siewerts klassifikasjon av svulter i den gastroøsofageale overgang er nødvendig for planlegging av behandling.
Type I: Sentrum av tumor proksimalt for den gastroøsofageale overgang – utgangspunkt i Barrett-slimhinne.
Type II: Omfatter den gastroøsofageale overgang, både proksimalt og distalt (den egentlige cardiacancer).
Type III: Lokalisert distalt for den gastroøsofageale overgang (magesekken).
Svulster i den gastroøsofageale overgang er nesten uten unntak adenokarsinomer. Type III-kreft klassifiseres som magesekkreft og behandles som slik med total gastrektomi og øsofagojejunostomi. Type I kreft klassifiseres som spiserørskreft og behandles med reseksjon av spiserør og (vanligvis) øsofagogastrostomi.
Type II kreft oppfattes nå av de fleste sentre som spiserørskreft og behandles som dette, men noen sentre vil fortsatt gjøre total gastrektomi og øsofagojejunostomi. En nasjonal registerstudie fra Nederland viste ingen signifikant forskjell i tre års overlevelse mellom øsofagusreseksjon og gastrektomi ved gastroøsofageale overgangssvulster, men viste at øsofagusreseksjon er vanligst (Jezerskyte et al., 2020). En større studie fra den amerikanske NCDB databasen (n=9594) ble publisert i 2021. I denne studien var fem års overlevelse etter øsofagektomi (53 %) bedre enn ved gastrektomi (45 %) ved Siewert type II svulster (p<0.001) (Kamarajah et al., 2021). Det pågår en internasjonal randomisert studie som sammenligner transtorakal øsofagektomi med transhiatalt utvidet gastrektomi ved Siewert type II svulster (Leers et al., 2020). Frem til resultatet fra denne studien foreligger anbefales primært øsofagektomi ved Siewert type II svulster.
Plateepitelkarsinomer behandles som spiserørskreft.
Valg av operasjonsmetode
Spiserøret kan fjernes kirurgisk med tilgang via brysthule (vanligvis høyresidig torakotomi) eller via mellomgulvet (transhiatalt) og jugulum. I en europeisk randomisert studie fant man ingen forskjell i 5-års overlevelse ved transtorakal versus transhiatal reseksjon ved distale adenokarsinomer. Ved Siewert type I-svulster var det imidlertid 14 % bedre overlevelse etter transtorakal reseksjon og utvidet to-felts lymfeknutedisseksjon sammenlignet med transhiatal tilgang (Omloo et al., 2007). Det er antatt at komplikasjonsfrekvens hos pasienter som blir opereret gjennom transhiatal tilgang, er lavere enn ved transtorakal tilgang, og denne tilgangen kan derfor anbefales hos gamle og skrøpelige pasienter (Hulscher & van Lanschot, 2005; Hulscher et al., 2002). Transhiatal øsofagusreseksjon kan gjøres laparoskopisk, med adekvat lymfadenektomi i nedre mediastinum. En kohortstudie fra Ohio State University (n=82) rapporterte 52 % fem års overlevelse ved denne tilgangen (Haisley, Abdelmoaty, & Dunst, 2021).
Ved adenocarcinom, og spesielt plateepitelcarcinom, foreligger det betydelig risiko for submukosal tumorutbredelse. Tumornær reseksjonskant kan således øke risiko for ufri rand og ha betydning for prognose. Det foreligger evidens for at en reseksjonsmargin for adenokarsinomer på mindre enn 3 cm, målt in vivo, har negativ prognostisk betydning (Barbour et al., 2007; Mine et al., 2013).
Operasjonen kan utføres åpent, thoracolaparoskopisk eller som en kombinasjon. Disse metodene er alle etablert i Norge.
Andelen mini-invasiv kirurgi forspiserørskreft er økende. En internasjonal undersøkelse i regi av ISDE (International Society for Diseases of the Esophagus) fra 2014 blant 1118 spiserørskirurger viste at 43 % brukte miniinvasiv teknikk, en betydelig økning fra rundt 14 % ved den forrige undersøkelsen fra 2007 (Haverkamp, Seesing, Ruurda, Boone, & R, 2017). To prospektive studier har rapportert lovende resultater i gruppen med total minimal invasiv og hybrid minimal invasiv reseksjon av spiserør sammenlignet med gruppen med åpen operasjon (Biere et al., 2012; Christophe Mariette et al., 2015). Disse studiene viste lengre operasjonstid, mindre blødning, færre lungekomplikasjoner, kortere sykehusopphold og bedre livskvalitet i grupppen med minimal invasiv kirurgi versus gruppen med åpen operasjon. Flere retrospektive og case-kontroll studier (D'Journo & Thomas, 2014; Wullstein, Ro-Papanikolaou, Klingebiel, Ersahin, & Carolus, 2015) har vist lignende resultater hvor pasientene i minimal invasiv gruppe kommer best ut i forhold til disse parametere. I 2019 ble det vist i en prospektiv randomisert studie at færre pasienter (34 %) i gruppen med hybid tilgang hadde alvorlige intraoperative og postoperative komplikasjoner sammenlignet med pasientene (64 %) i gruppen med åpen tilgang (C. Mariette et al., 2019). Minimal invasiv operasjon er teknisk krevende, og dokumentasjon så langt (D'Journo & Thomas, 2014; Wullstein et al., 2015) tilsier at dette ikke gir bedre langtidsoverlevelse enn ved konvensjonell åpen kirurgi. Den franske randomiserte MIRO studien (n=207) viste en nær-signifikant bedret overlevelse ved hybrid versus åpen tilgang, også ved langtidsoppfølging (59 % versus 47 % fem års overlevelse, p=0.09). Man antar at denne forskjellen kan tilskrives mindre lungekomplikasjoner ved hybrid tilgang (C. Mariette et al., 2019). Det er lite evidens for at total minimal invasiv øsofagusreseksjon (TMIE) er bedre en hybrid minmal invasiv øsofagusreseksjon (HMIE). En metaanalyse som inkluderte 29 studier, hvor ingen var randomiserte, viste lavere morbiditet ved TMIE versus HMIE, men også færre høstede lymfeknuter, hyppigere anastomoselekkasje og lengre operasjonstid (van Workum, Klarenbeek, Baranov, Rovers, & Rosman, 2020). Miniinvasiv tilgang supplert med robotassistert teknikk ble beskrevet i 2003 (Horgan, Berger, Elli, & Espat, 2003), og i perioden 2007–2017 er 491 operasjoner fra 12 studier rapportert i en metaanalyse (Taurchini & Cuttitta, 2017). Fordeler med denne teknikken gjelder særlig i torakale del av operasjonen. På grunn av bedre tilgang, bruk av leddede instrumenter og eliminasjon av fingertremor blir det lettere å sy intratorakal anastomose og å utføre presis lymfeknutedisseksjon langs nerver og luftveier. Det er også ergonomisk en fordel for kirurgen å kunne sitte i mer fysiologisk stilling i en konsoll under inngrepet sammenlignet med uten assistanse fra robot. Derfor er det sannsynlig at robotassistert teknikk ved miniinvasiv reseksjon raskt vil øke i omfang. En populasjonsbasert studie fra USA basert på en nasjonal kreftdatabase for perioden 2010–2012, viste at 30.7 % fikk minimal invasiv reseksjon av spiserør og innen denne gruppen fikk 17.6 % robotassistert reseksjon (Yerokun et al., 2016).
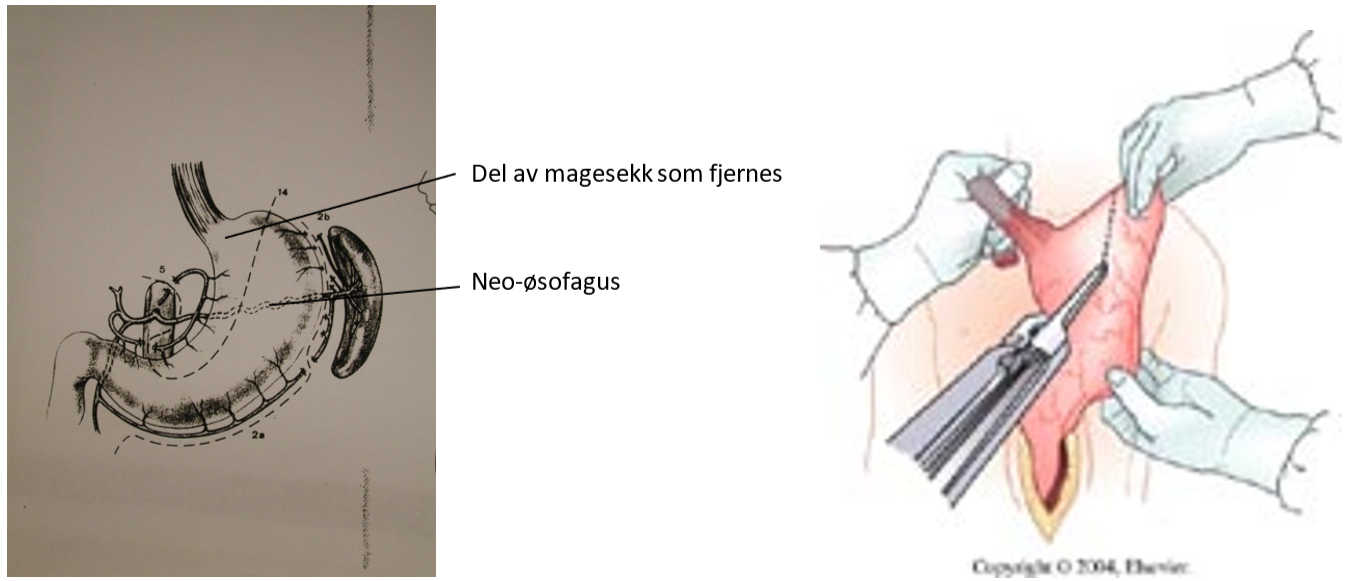
Som rekontruksjon etter øsofagektomi konstrueres oftest et rør av magesekken, som anastomoseres til proksimale del av spiserøret, enten i øvre del av thorax eller på halsen. Som alternativ til magesekk kan man også bruke colon som substitutt for spiserøret. Et tredje alternativ er tynntarm, som rekker opp til carina. Ved svulster lokalisert i cervikale del av spiserøret, kan man erstatte denne delen med et fritt transplantat av tynntarm. I Norge behandles svulster i cervikale øsofagus nærmest utelukkende med radiokjemoterapi.
Lymfeknutedisseksjon ved spiserørskreft
Sist faglig oppdatert: 28.06.2022
Spredningsmønster
Metastaser til lymfeknuter er vanlig ved kreft i spiserøret og nært korrelert til T-stadium og prognose (Ovrebo, Lie, Laerum, Svanes, & Viste, 2012). Andelen pasienter med påviselige lymfeknutemetastaser etter kirurgi er 51 %. Denne andelen øker til 71 % når lymfeknutene også undersøkes immunhistokjemisk (Hosch et al., 2001). "Skipmetastaser" (metastaser til anatomisk fjerne lymfeknuter uten metastaser til tumornære lymfeknuter) kan sees hos 66 % når lymfeknuteneundersøkes med immunhistokjemi. Den prognostiske betydningen av slike "skipmetastaser" er imidlertid usikker (Hagens, van Berge Henegouwen, Cuesta, & Gisbertz, 2017). Lokalisasjon og forekomst av lymfeknutemetastaser varierer, avhengig av primærtumors lokalisasjon, histologi, T-stadium og evt. neoadjuvant behandling. I flere publikasjoner er det også vist en sammenheng mellom tumors utbredelse i lengde og forekomst av lymfeknutemetastaser for både plateepitelcarcinom og adenocarcinom (Harada et al., 2021; Koyanagi et al., 2018; Kurokawa et al., 2015). Kirurgisk strategi og valg av evt. neoadjuvant/perioperativ behandling bestemmes delvis av spredningsmønsteret, men det er fortsatt ingen internasjonal konsensus vedrørende hvor omfattende lymfeknutedisseksjonen skal eller bør være (van Rijswijk, Hagens, van der Peet, van Berge Henegouwen, & Gisbertz, 2019). Spesielt gjelder dette for adenocarcinomer som utgjør 85 % av alle svulstene i den vestlige verden. Forskjellige klassifikasjonssystemer for lymfeknutestasjoner og forskjellig forekomst av plateepitelcarcinom og adenocarcinom i Asia og Vesten gjør det dessuten vanskelig å sammenligne data både mht. forekomst av lymfeknutemetastaser i forskjellige stasjoner og betydningen av kirurgisk strategi for lymfeknutedisseksjon. En pågående internasjonal cohortstudie (TIGER study) med 50 deltagende sentra i 18 land har som hensikt å beskrive fordelingen av lymfeknutemetastaser hos pasienter med resektabel kreft i øsofagus eller gastroøsofageale overgang med mål om å utvikle et internasjonalt, enhetlig klassifikasjonssystem for å optimalisere kirurgisk strategi (Hagens et al., 2019). Studien kan muligens også avklare prognostisk verdi av glandelmetastaser i forskjellige stasjoner.
Spredning i lymfebaner skjer langs spiserøret til regionale og ikke-regionale lymfeknuter i et submucøst nettverk, men også gjennom muskularis propria til regionale lymfeknuter og til fjerne lymfeknuter via ductus thoracicus og det venøse system. Vanlige lymfeknutestasjoner for spredning er på hals, i mediastinum og i abdomen, og både plateepitelcarcinom og adenocarcimom kan spre seg til alle stasjoner med noe forskjellig frekvens avhengig av faktorer som nevnt foran. Spredningsmønsteret er mye bedre undersøkt og beskrevet for plateepitelcarcinom (dominerende tumorform i Asia) enn for adenocarcinom (dominerende tumorform i Vesten) (Cense, van Eijck, & Tilanus, 2006).
Lymfeknutestasjoner ved spiserørskreft
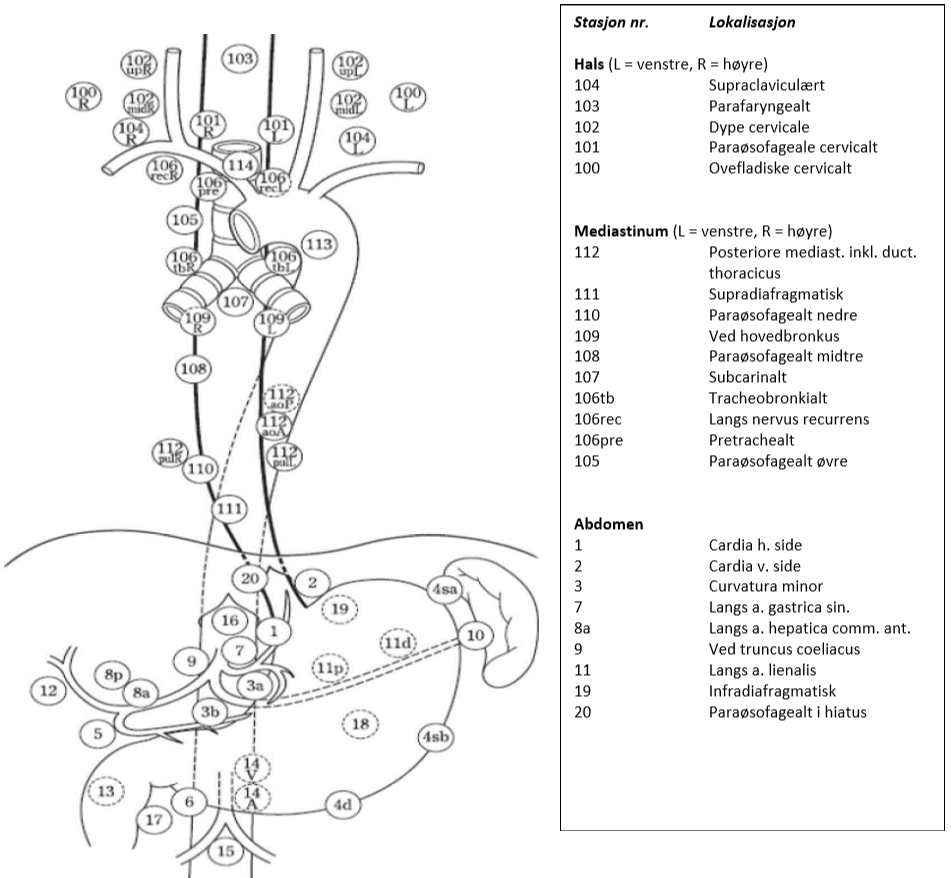
Figuren viser aktuelle glandelstasjoner ved spiserørskreft i hht. Japanese Esophageal Society (JES) klassifikasjon, 11. utgave (Japanese Classification of Esophageal Cancer, 11th Edition: part I, 2017). Fordelen med denne klassifikasjonen i forhold til f.eks. AJCC (American Joint Committee on Cancer), som er en annen ofte brukt klassifikasjon, er at den også innbefatter lymfeknuter på halsen og dermed beskriver lymfeknutestasjoner i alle tre aktuelle områder (hals, mediastinum, abdomen). I tillegg brukes samme nummerering av stasjonene som benyttes ved kreft i magesekken.
I hht. UICC. 8 utgave defineres regionale lymfeknuter ved spiserørskreft, uavhengig av primærtumors lokalisasjon, som lymfeknuter i drenasjeområdet for spiserøret, inklusive lymfeknuter ved truncus coeliacus og paraøsofageale lymfeknuter på halsen, men ikke supraclaviculære lymfeknuter (Brierley et al., 2017).
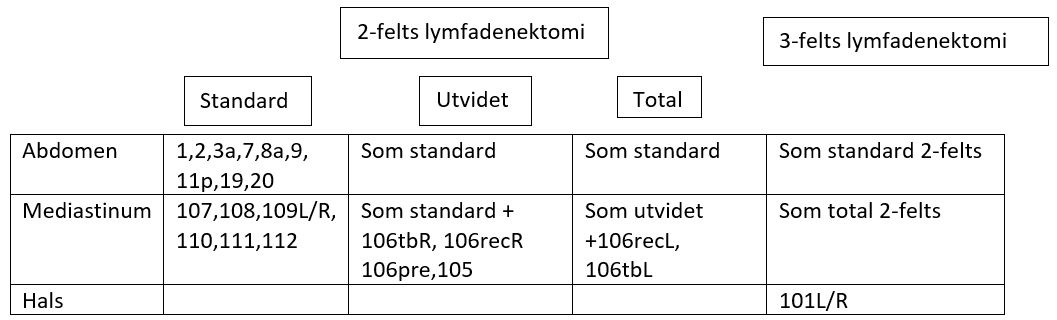
2-/3-felts Lymfeknutedisseksjon
Ved spiserørskreft skiller man mellom 2-felts og 3-felts lymfeknutedisseksjon. 2-felts disseksjon innbefatter lymfeknutestasjoner i øvre del av abdomen og i mediastinum, mens det ved 3-felts disseksjon også tas med lymfeknutestasjoner på halsen. Begrepene er imidlertid ikke absolutt entydige. For eksempel vil noen kreve disseksjon av alle stasjoner på halsen (101-104) i 3-felts konseptet, mens andre nøyer seg med stasjon 101 og 104 (selv om det bare er stasjon 101 som defineres som regional i hht. UICC 8. utgave). På tilsvarende måte er det innarbeidet en generell praksis på at stasjon 8a og 11p også tas med i den abdominale disseksjonen, selv om disse stasjonene heller ikke defineres som regionale i hht. UICC 8. utgave. I tillegg inndeles den mediastinale lymfeknutedisseksjonen gjerne i standard, utvidet og total, uten at litteraturen heller her er samstemt mht. hvilke glandelstasjoner som inngår i de forskjellige begrepene.
International Society for the Diseases of the Esophagus (ISDE) definerte ved en konsensuskonferanse i 1995 (Fumagali, 1996) de forskjellige typene av 2-felts disseksjon på følgende måte:
Standard 2-felts lymfadenektomi omfatter, i tillegg til en vid eksisjon av primærtumor, også lymfadenektomi av hele posteriore mediastinum fra diafragma opp til og med de subcarinale lymfeknuter og opp til og med det aortopulmonale vindu. I abdomen inkluderes lymfeknuter langs truncus coeliacus, a. hepatica communis og a. lienalis samt lymfeknuter langs curvatura minor av ventrikkelen og i omentum minus.
Utvidet 2-felts lymfadenektomi omfatter, i tillegg til stasjoner som inngår i standard 2-felts disseksjon, også lymfeknuter paratrachealt på høyre side opp til og med lymfeknutene langs høyre nervus recurrens og truncus brachiocephalicus.
Total 2-felts lymfadenektomi omfatter, i tillegg til stasjoner som inngår i standard og utvidet 2-felts disseksjon, også lymfeknuter paratrachealt på venstre side og langs venstre nervus recurrens.
I en internasjonal spørreundersøkelse blant 50 erfarne øsofaguskirurger, de fleste fra høyvolumsentra, var det meget stor variasjon i hvilke lymfeknutestasjoner som ble fjernet ved både plateepitelcarcinom og adenocarcinom i forskjellige lokalisasjoner. Andelen kombinasjoner av forskjellige lymfeknutestasjoner som ble fjernet ved sammenlignbare svulster var høy, mens antallet kirurger som fjernet nøyaktig den samme kombinasjon av lymfeknutestasjoner var lav (van Rijswijk et al., 2019).
Tabellen under reflekterer et forsøk på begrepsavklaring basert på hvordan lymfeknutedisseksjonen oftest beskrives og inndeles i hht. stasjons nr. etter JES klassifikasjon.
Figuren under illustrerer lymfeknutedisseksjonen (unntatt utvidet 2-felts) på en annen måte etter Matsuda et al. (Matsuda, Takeuchi, Kawakubo, & Kitagawa, 2017).
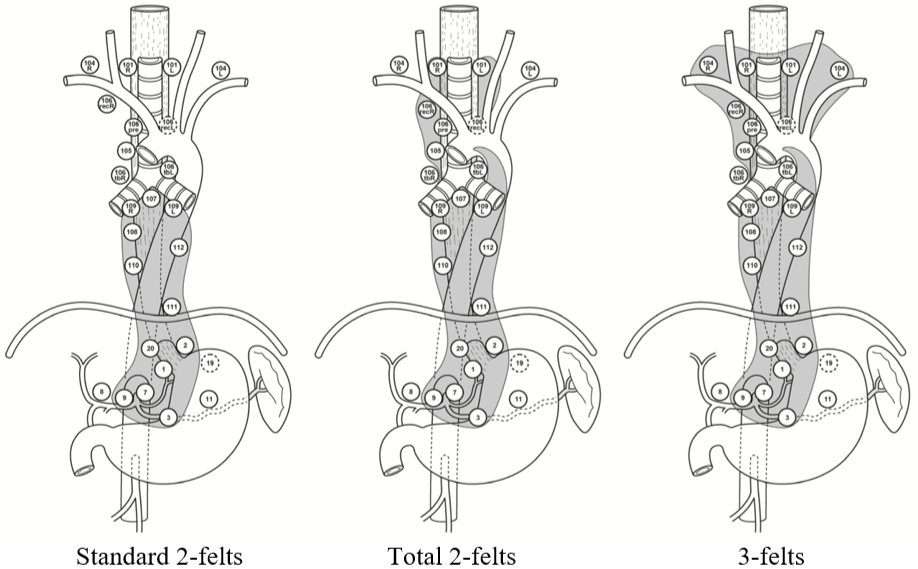
I hht. den vanligste forståelsen av begrepene vil utvidet 2-felts disseksjon være som total unntatt stasjon 101, 106recL og 106tbL. Figuren reflekterer også at det i asiatiske land er vanlig å ta med stasjon 104 i halsglandeldisseksjonen, mens i vesten oppfattes dette (st. 104) som fjernmetastaser med konsekvens irresektabilitet.
Lymfeknutedisseksjon ved plateepitelkarsinom
Plateepitelkarsinom opptrer i alle områder av spiserøret med risiko for spredning til lymfe-knuter på hals, i mediastinum og i abdomen, og tumors lokalisasjon påvirker sprednings-mønsteret.
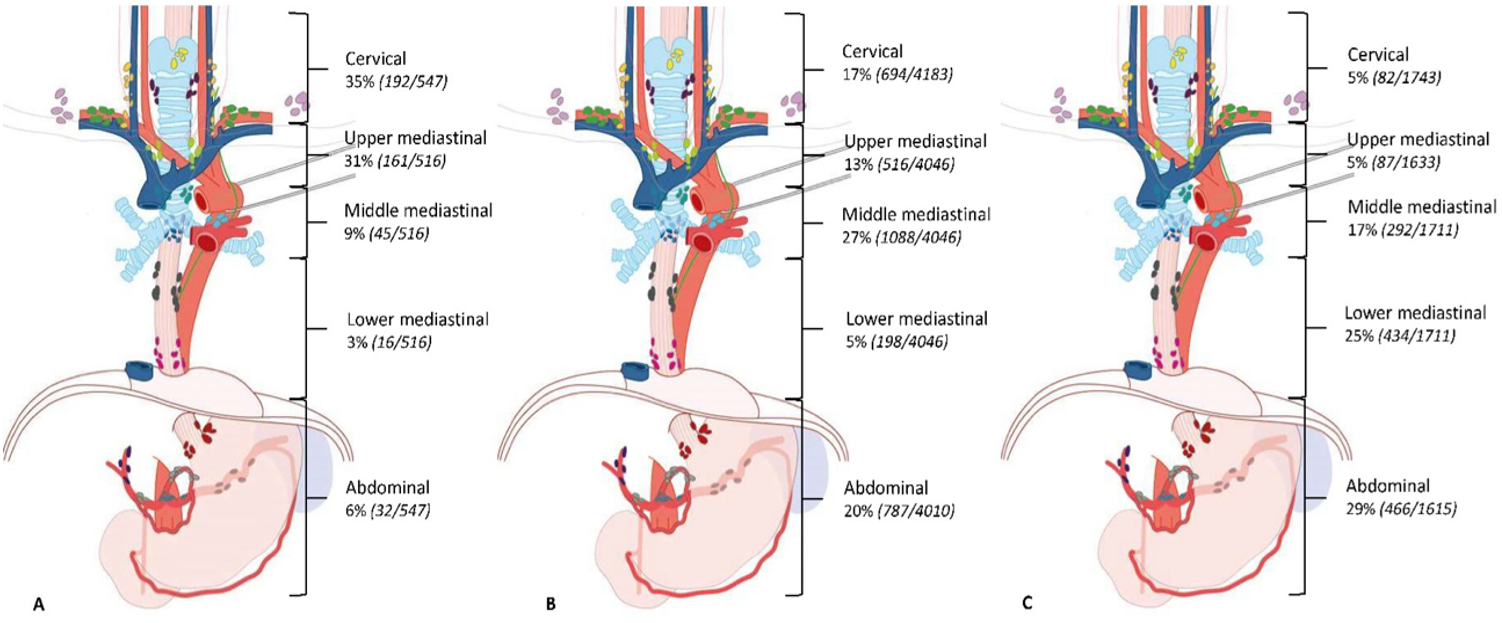
Figuren over viser prevalens av lymfeknutemetastaser per tumorlokalisasjon hos 7.982 pasienter med plateepitelcarcinom som gjennomgikk primær kirurgi, presentert som % av pasienter med metastaser i de forskjellige regioner. Av i alt 8.543 pasienter med plateepitelcancer i denne studien var primærtumors lokalisasjon: A = Øvre thorakale øsofagus (n= 726, 9 %), B = Midtre thorakale øsofagus (n= 5.130, 60 %), C = Nedre thorakale øsofagus (n= 2.687, 31 %) (Hagens, van Berge Henegouwen, & Gisbertz, 2020).
Verdt å merke seg fra denne studien var også følgende; hos pasienter med svulst i øvre thorakale øsofagus var lymfeknutespredning oftest sett langs høyre nervus recurrens (60 %) og cervicale paraøsofageale glandler (høyre 34 % og venstre 22 %). Hos pasienter med svulst i midtre thorakale øsofagus var prevalensen av lymfeknutespredning høyest langs høyre nervus recurrens (23 %), cervicale paraøsofageale glandler på høyre side (24 %) og midtre paraøsofageale glandler (23 %). Metastastiske lymfeknuter langs a. gastrica sinistra (28 %) og nedre thorakale øsofagus (23 %) hadde høyest prevalens hos pasienter med svulster i nedre thorakale øsofagus.
Med bakgrunn i utbredt lymfeknutespredning ved plateepitelkarsinom finnes det støtte for 3- felts lymfeknutedisseksjon ved denne kreftformen, særlig ved svulster i midtre og øvre del av spiserøret, men omkostningen er gjerne økt postoperativ morbiditet og redusert livskvalitet. I en metaanalyse av 2 RCT + 18 observasjonsstudier med 7.980 pasienter som hadde gjennomgått R0 reseksjon ble det funnet overlevelsesgevinst etter 3-felts disseksjon. Det er ikke redegjort for nøyaktig andel pasienter med plateepitelcarcinom i analysen annet enn at "nesten alle" var plateepitelcarcinomer. Relativ risiko (RR) for død var høyere for to-felts lymfeknutedisseksjon etter ett år (1.16, 95 % CI: 1.09 – 1.24), 3 år (1.44, 95 % CI: 1.19 – 1.75) og 5 år (1.37, 95 % CI 1.18 – 1.59) (Ma et al, WJS 2014, 20(47):18022-18030.Three-field vs two-field lymph node dissection for esophageal cancer: A meta-analysis). I gruppen med 3-felts disseksjon var det signifikant hyppigere forekomst av stemmebåndsparese og anastomoselekkasje. Forfatterne konkluderer imidlertid med at det er vanskelig å trekke definitiv konklusjon av studien pga. stor heterogenisitet mht. resultater i studiene som inngikk i analysen.
Lymfeknutedisseksjon ved adenokarsinom
Adenocarcinom er nært lokalisert til overgangssonen mellom plateepitel og sylinderepitel (GEJ), og kirurgisk behandling er tradisjonelt styrt etter tumors origo og Siewerts og Nishis klassifisering. Utbredelsen av lymfeknutemetastaser fra adenokarsinom er ikke like godt kartlagt som ved plateepitelcarcinom i spiserøret. I mange tilfeller er det avstått fra lymfeknutedisseksjon i øvre mediastinum hos pasienter med adenocarcinom i distale del av spiserøret. Det reduserer kunnskapen om utbredelse av lymfeknutemetastaser i øvre mediastinum.
Tradisjonelt har det vært argumentert for at lymfeknutedisseksjon i øvre mediastinum differensieres for plateepitelcarcinom og adenocarcinom. I en studie av Mine et al (Mine et al., 2019) ble lymfeknutespredningen kartlagt blant 69 pasienter med adenocarcinom (Siewert I eller II) og 73 pasienter med plateepitelcarcinom ved GEJ som samtidig affiserte mer enn 30 mm av esophagus og var operert med åpen eller thorakoskopisk øsofagektomi. Figuren under viser at det ikke er vesentlig forskjell mellom plateepitel- og adenocarcinom for utbredelsen av lymfeknutemetastaser i mediastinum. Studien viste også at andelen med lymfeknutemetastaser i øvre mediastinum var relativ høy.
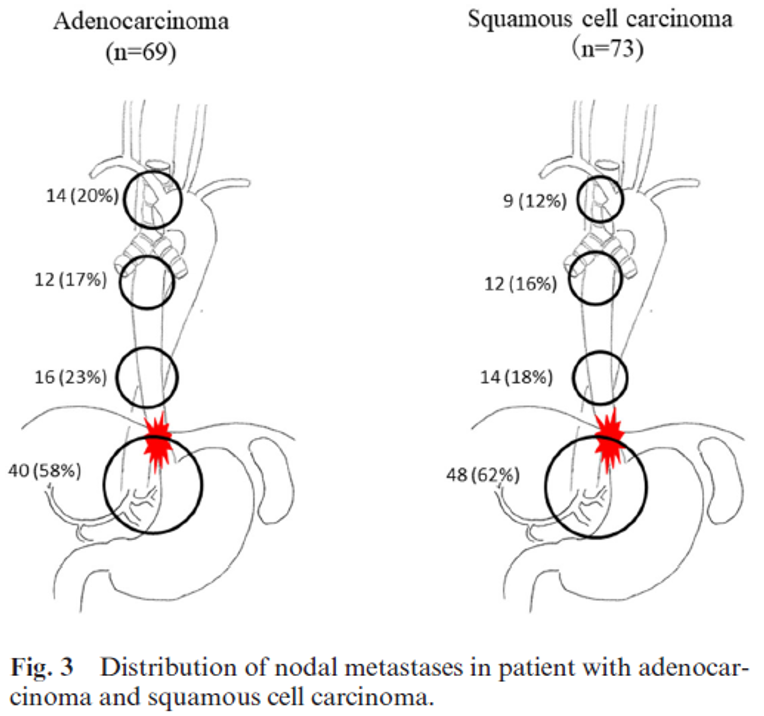
Tumor klassifisering i hennhold til Siewert kan være vanskelig, men er rådgivende for kirurgisk prosedyre og sannsynlighet for frie reseksjonsmarginer. Den er imidlertid ikke nødvendigvis den beste metoden for bestemmelse av lymfeknutedisseksjon. Den nære sammenhengen mellom lengden av tumor over GEJ kan være en viktig prediktor for hvilke lymfeknutestasjoner som kan inneholde metastaser i mediastinum, og kanskje bør det utføres lymfeknutedisseksjon i øvre mediastinum blant alle med mer enn 25 mm distal øsofagusaffeksjon.
Lymfeknutedisseksjon - Oppsummering
Lymfeknutedisseksjon er viktig for kirurgisk stadieinndeling og lokoregional kontroll av kreftsykdom. Ekstensiv lymfeknutedisseksjon ved spiserørskreft synes å bedre både total og sykdomsfri overlevelse, (Visser, Markar, Ruurda, Hanna, & van Hillegersberg, 2019), og i en stor studie på 3.572 pasienter som gjennomgikk R0 reseksjon for adenocarcinom (60 %) eller plateepitelcarcinom (40 %) var et høyt antall lymfeknuter i resektatet en uavhengig prognostisk faktor for bedret overlevelse etter primær kirurgi (Rizk et al., 2010). Andre studier og metaanalyser har imidlertid betvilt denne konklusjonen (Nafteux, Depypere, Van Veer, Coosemans, & Lerut, 2017). I CROSS-studien hadde f.eks. antall reseserte lymfeknuter etter primær kirurgi en positiv korrelasjon med overlevelse, mens dette ikke var tilfellet hos de som fikk neoadjuvant behandling. Det er derfor ikke entydig hva som er nødvendig eller tilstrekkelig lymfeknutedisseksjon ved kreftsykdom i spiserøret. Komplett disseksjon av alle lymfeknuter tilhørende spiserøret innebærer 3-felts lymfeknutedisseksjon, dvs. i abdomen, mediastinum og på hals. Metoden er omdiskutert og ikke vanlig standard i Europa på grunn av økt postoperativ morbiditet, redusert livskvalitet og usikker overlevelsesgevinst, det siste muligens pga. den negative effekt på onkologisk effekt som postoperative komplikasjoner kan medføre. Moderne neoadjuvant/perioperativ behandling øker lokoregional sykdomskontroll, bedrer total overlevelse og gir sannsynligvis den optimale balansen mellom nytte og risiko uten de komplikasjonene en cervical lymfeknutedisseksjon fører med seg. Effekten av moderne neoadjuvant/perioperativ behandling kan dessuten tenkes å få den konsekvensen at man i fremtiden baserer sin lymfeknutedisseksjon på lymfeknutestatus ved ny utredning etter preoperativ behandling og ikke på lymfeknutestatus ved primærutredningen (Hagens et al., 2020).
I tillegg kommer muligens vaktpostlymfeknute konseptet til å ha betydning i fremtiden. Vaktpostlymfeknute defineres som første lymfeknute(r) som mottar lymfatisk drenasje fra stedet for primærtumor. Dersom vaktpostlymfeknute kan lokaliseres og er uten patologisk påvisbart tumorvev kan man tenke seg både en begrenset reseksjon av primærtumor, men særlig en begrenset lymfeknutedisseksjon med redusert postoperativ mortalitet/morbidet og bedret livskvalitet som følge (Takeuchi & Kitagawa, 2019). Allerede i dag anbefaler noen å bruke lymfeknuterved høyre nervus reccurens som vaktpostlymfeknute., spesielt ved plateepitelcarcinom (Shiozaki et al., 2001; Wang et al., 2020; Yuan & Mao, 2019).
Lymfeknutedisseksjon – Anbefaling
Basert på foreliggende kunnskap er det vanskelig å definere en enhetlig og evidensbasert anbefaling vedrørende omfanget av lymfeknutedisseksjon ved spiserørskreft. Stategi for lymfeknutedisseksjon vil dessuten være nært knyttet til strategi for reseksjon av primærtumor og lokalisasjon av anastomose (intrathorakalt eller på hals). En pragmatisk tilnærming kan være:
- Ved thorakal anastomose gjøres en standard 2-felts lymfadenectomi uavhengig av histologi
- Ved cervical anastomose uten mistanke om lymfeknutemetastaser cranialt for carina gjøres en standard 2-felts lymfadenektomi ved adenocarcinom i distale og midtre del av spiserøret.
- Ved adenocarcinom i øvre del og ved plateepitelcarcinom (uavhengig av lokalisasjon) samt ved mistanke om lymfeknutemetastaser cranialt for carina gjøres en utvidet eller total 2-felts lymfadenektomi.
- Dersom det preoperativt påvises cervicale lymfeknutemetastaser eller intraoperativt påvises metastaser langs nervus recurrens (evt. med frysesnitt) vurderes det å gjøre 3-felts lymfadenektomi.
Postoperativt regime
Sist faglig oppdatert: 28.06.2022
Postoperativt kan pasienten begynne å drikke etter kort tid. Tidlig enteral ernæring via jejunumkateter eller nasoduodenal sonde reduserer postoperativ morbiditet (lungekomplikasjoner og anastomoselekkasje) (Peng, Cai, Niu, & Chen, 2015) . En amerikansk registerstudie viste at jejunumkateter ikke øker antall reinnleggelser, men er assosiert med lavere mortalitet (Zheng et al., 2021). Med epidural smertelindring kan pasientene mobiliseres nærmest umiddelbart. ERAS protokoller for perioperativ behandling benyttes ved stadig flere sentre.
Anbefaling
Etter reseksjon av spiserøret bør pasientene få tidlig enteral ernæring (evidensgrad B).
Komplikasjoner
Sist faglig oppdatert: 28.06.2022
Totalt finner man komplikasjoner hos 30–65 % etter reseksjon av spiserøret. De hyppigste komplikasjonene er pneumoni hos inntil 30 % og plevravæske. Anastomoselekkasje forekommer i 1–24 %, lymfelekkasje 1–2 % og recurrensparese 1–2 %. En metaanalyse viste lavere forekomst av anastomoselekkasje ved såkalt prekondisjonering av magesekkrøret. Det foreligger imidlertid ingen randomiserte studier og prekondisjonering er foreløpig lite utbredt (Michalinos et al., 2020). Sykehusmortalitet er rapportert fra 2–10 %, bør være under 5 %. Ved bruk av magesekk som spiserørsubstitutt er en del pasienter plaget med magesekkretensjon. Det er lite dokumentasjon for at pyloruplastikk reduserer postoperativ interpronatretensjon (Cheung, Siu, & Wong, 1987) .
Patologi – Besvarelse av spiserørsresektater
Sist faglig oppdatert: 28.06.2022
Opplysninger på remissen som følger preparatet
- Neoadjuvant behandling: ja/nei
- Hva resektatet omfatter: bare spiserøret eller spiserøret og del av magesekk
- Tumors lokalisasjon
- Innhold i eventuelle separate glass
Håndtering av preparatet før fiksering
Oppklipping av resektatet og oppspenning på korkplate. Dersom resektatet består av både spiserøret og magesekk, bør magesekken klippes opp langs curvatura major og langs stiftede reseksjonsrender.
Håndtering av preparatet etter fiksering
Det anbefales å fotografere resektatet og tegne inn/markere snittuttak.
Snittuttak utføres etter anbefaling i «Veileder i biopsibesvarelse av maligne svulster»
Besvarelse fra patologen bør inneholde følgende opplysninger
- Tumors lokalisasjon, bare i spiserøret eller både i spiserøret og magesekk, evt. hvor stor andel av tumor er lokalisert i magesekk
- Tumors histologiske type, plateepitelkarsinom, adenokarsinom eller annet
- Mikrosatelitt instabilitet (MSI) eller «mismatch repair protein» (MMR), PD-L1 status etter CPS- og eventuelt TPS-gradering og Epstein-Barr virus (EBV) undersøkes etter forespørsel fra kliniker
- Differensieringsgrad
- Tumors største diameter på overflaten
- Infiltrasjonsdybde
- Avstand til proksimale og distale reseksjonsrand
- Avstand til sirkumferent reseksjonsrand i spiserøret, evt. langs curvatura major og/eller minor i magesekk
- Tumorinfiltrasjon i kar/perinevralt
- Antall lymfeknuter med metastaser/totalt antall lymfeknuter
- Regresjonsgrad etter neoadjuvant behandling (ulike klassifiseringssystemer, eks. CAP (Burgart, Chopp, & Jain, 2021))
- SNOMED/NORPAT-kode
- TNM-klassifisering (8. utgave) (Brierley et al., 2017)
Konferer for øvrig «Veileder i biopsibesvarelse av maligne svulster. 3. utg. (versjon 5.0 – 2018) Oslo: Den norske patologforening; 2018» (Den norske patologforening, 2018).
Onkologisk behandling
Sist faglig oppdatert: 28.06.2022
Neoadjuvant/adjuvant behandling
Plateepitelkarsinom (PEK) vs. Adenokarsinom (AK)
Det er som kjent to hovedformer av spiserørskreft, plateepitelkarsinom (PEK) og adenokarsinom (AK). Historisk har disse blitt behandlet likt, og kliniske studier skilte tidligere ikke mellom dem. Dog er det økende kunnskap om at disse skiller seg både hva gjelder patogenese, epidemiologi, tumorbiologi og prognose.
«The Cancer Genomic Atlas research network» har utført omfattende molekylære analyser på 164 spiserørssvulster, 359 magesekk AK og 36 overgangs AK. De viste bl.a. at PEK i spiserør lignet mer på PEK i andre organ enn de lignet på AK i spiserør. AK i spiserør hadde en liknende fenotype som den kromosomalt instabile varianten av magesekkreft (Cancer Genome Atlas Research et al., 2017). Det er enighet om at gastroøsofageal kreft ikke lenger kan ses på som en enkelt sykdom, og de fleste av dagens studier rekrutterer ikke lenger en blanding av AK og PEK, spesielt fordi PEK er mer strålefølsom.
Likevel er det fortsatt uavklart hvordan histologien skal styre behandlingsstrategien. Et PEK i spiserør som går i komplett remisjon på radiokjemoterapi vil noen vurdere å observere uten kirurgi, mens de færreste vil anbefale dette for et AK i spiserør. Videre er det ikke avklart hvilken strategi som er best i forhold til et resektabelt AK i spiserør; neoadjuvant radiokjemoterapi, neoadjuvant kjemoterapi eller perioperativ kjemoterapi.
Det har skjedd en stor utvikling i behandling av spiserørskreft de siste to tiårene både hva gjelder kirurgiske teknikker og onkologisk behandling. Med unntak av de tidligste stadier vil behandlingen som oftest være multimodal.
Begrenset sykdom (cT1 cN0 M0)
Ved begrenset sykdom er kirurgi alene, å foretrekke (se kirurgikapittel over). Nytten av neoadjuvant onkologisk behandling ved begrenset sykdom er usikker, siden antallet av pasienter med begrenset sykdomsom har blitt inkludert i prospektive randomiserte studier er lavt. En fase III studie fra 2014 randomiserte 195 pasienter med spiserørskreft i stadium I og II til neoadjuvant radiokjemoterapi før kirurgi eller kirurgi direkte. Det forelå ingen 3 års overlevelsesforskjell, den postoperative mortalitet var høyere med neoadjuvant behandling, og studien ble derfor stoppet. Neoadjuvant radiokjemoterapi besto i 45 Gy fordelt på 25 fraksjoner over 5 uker, og to CiFu-kurer konkomitant (C. Mariette et al., 2014).
For de pasientene som ikke ønsker kirurgi eller er medisinsk inoperable, kan definitiv radiokjemoterapi vurderes (se under).
cT2 cN0 M0
Verdien av neoadjuvant behandling ved cT2N0M0 er usikker. Studier og metaanalyser er ofte av begrenset verdi pga. lite antall pasienter, heterogenisitet mht. tumortyper, stråledoser, kjemoterapiregimer, preoperativ staging og kirurgisk radikalitet (C. Mariette et al., 2014). Flere studier har ikke vist bedret overlevelse sammenlignet med kun kirurgi (Dolan et al., 2016; C. Mariette et al., 2014; Sheraz R. Markar et al., 2016; Mota et al., 2018; Speicher et al., 2014), og i en av studiene (C. Mariette et al., 2014) påviste man signifikant økt postoperati mortalitet i kombinasjonsarmen. Preoperativ presisjon mht. klinisk stadium er beheftet med usikkerhet både når det gjelder cT-, men særlig cN-stadium. I flere av de nevnte materialene (Dolan et al., 2016; C. Mariette et al., 2014; Sheraz R. Markar et al., 2016) viste det seg ved histologisk undersøkelse av resektat å foreligge pN+ i > 50 % av tilfellene til tross for at preoperativ staging tilsa cN0. Dette hadde likevel ikke betydning for overlevelse. I en retrospektiv gjennomgang av et større materiale fra amerikanske National Cancer Data Base fant man at det var bedre median total overlevelse hos pasienter som fikk neoadjuvant behandling, uavhengig av senere patologisk stadium, enn hos de som gikk til kirurgi direkte og som fikk oppjustert patologisk stadium i forhold til klinisk stadium (Samson et al., 2016). Nytten ved neoadjuvant behandling ved cT2N0M0 er derfor i øyeblikket uklar. Sett i lys av resultatene fra CROSS-studien (Shapiro et al., 2015) og usikkerheten ved klinisk staging, med høy grad av understaging, anbefales neoadjuvant behandling til pasienter med cT2N0M0 spiserørskreft.
Lokalavansert sykdom (cT2–4 eller cN1–3 M0)
Flere studier og metaanalyser har vist at neoadjuvant behandling med enten kjemoterapi eller radiokjemoterapi øker R0 reseksjonsraten og bedrer overlevelsen (Allum, Stenning, Bancewicz, Clark, & Langley, 2009; Kidane, Coughlin, Vogt, & Malthaner, 2015; Kranzfelder, Schuster, Geinitz, Friess, & Buchler, 2011; Pasquali et al., 2017; Ronellenfitsch, Schwarzbach, Hofheinz, Kienle, Kieser, Slanger, Burmeister, et al., 2013; Ronellenfitsch, Schwarzbach, Hofheinz, Kienle, Kieser, Slanger, & Jensen, 2013; Shapiro et al., 2015; Sjoquist et al., 2011; van Hagen et al., 2012; Y. Xu, Yu, Chen, & Mao, 2012; Zhou et al., 2020). Det finnes ikke nyere dokumentasjon for overlevelsesgevinst etter neoadjuvant radioterapi (uten cytostatika) og kirurgi sammenlignet med kirurgi alene (Malthaner, Collin, & Fenlon, 2006; Sjoquist et al., 2011).
I en fase II studie (Klevebro et al., 2016) er det funnet høy forekomst av histologisk komplette remisjoner både etter neoadjuvant kjemoterapi (9 %) og etter radiokjemoterapi (28 %). Tidligere studier har vist at pasienter med komplett remisjon har bedre overlevelse (Stockeld et al., 2001; Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial, 2002). Neoadjuvant onkologisk behandling er i dag ansett som standardbehandling ved resektabel, lokalavansert spiserørskreft.
Plateepitelkarsinom (PEK)
Metaanalyser og en fase III studie viser at pasienter med lokalavansert sykdom har nytte av preoperativ kjemoterapi, og sannsynligvis enda større nytte av preoperativ radiokjemoterapi, sammenlignet med kirurgi alene (Y. Huang et al., 2017; Kranzfelder et al., 2011; Sjoquist et al., 2011; van Hagen et al., 2012). Internasjonalt anbefales neoadjuvant radiokjemoterapi framfor neoadjuvant kjemoterapi ved resektabelt PEK.
Den nederlandske CROSS studien randomiserte 368 pasienter med resektabelt, lokalavansert kreft i spiserøret eller gastroøsofageal-overgang (GEJ) (klinisk stadium T1N1M0 eller T2–3N0–1M0 etter TNM 6) til enten neoadjuvant radiokjemoterapi og kirurgi eller til kirurgi alene. 75 % av pasientene hadde AK og 23 % hadde PEK. Man fant signifikant forlenget 5-års overlevelse i kombinasjonsarmen sammenlignet med kirurgi alene armen (47 % vs. 34 %) (van Hagen et al., 2012). Det var dobbelt så høy median overlevelse i kombinasjonsarmen sammenliknet med kirurgi alene (49 vs. 24 mnd.). Videre fant man en patologisk komplett respons (pCR) hos 29 % i kombinasjonsarmen. Blant AK var det 23 % pCR, og blant PEK var det 49 % pCR. Subgruppeanalyser viste størst gevinst av den neoadjuvante radiokjemoterapien for pasienter med PEK i spiserøret, og en noe mindre, men likevel signifikant overlevelsesgevinst for pasienter med AK.
I en oppdatert analyse av CROSS studien har det kommet 8 års overlevelsesdata (Shapiro et al., 2015). Denne viste for PEK en median overlevelse på 81,6 mnd. vs. 21,1 mnd. (HR 0,48) til fordel for kombinasjonsarmen. For AK var median overlevelse på 43,2 mnd. vs. 27,1 mnd. (HR 0,73) til fordel for kombinasjonsarmen.
På bakgrunn av denne og tidligere studier er rutinen i Norge i dag å gi neoadjuvant radiokjemoterapi ved lokalavansert, resektabel PEK og AK i spiserøret etter CROSS-regimet (41,4 Gy fordelt på 23 fraksjoner og konkomitant 5 ukentlig paclitaxel-karboplatin kurer).
To prospektivt, randomisert kontrollerte studier som sammenlignet definitiv radiokjemoterapi vs. en trimodal behandlingsstrategi med neoadjuvant radiokjemoterapi etterfulgt av kirurgi viste ingen forskjell i overlevelse, men man fant bedre lokal kontroll og mindre behov for palliative prosedyrer når kirurgi var del av den multimodale behandling (Bedenne et al., 2007; Stahl et al., 2005). Således kan definitiv radiokjemoterapi etterfulgt av tett oppfølging og vurdering for kurativ kirurgi ved behov være et alternativ, men en slik strategi bør fortrinnsvis være i regi av en studie. Vi har per i dag ingen data som sammenligner neoadjuvant radiokjemoterapi + kirurgi vs. definitiv radiokjemoterapi og kurativ kirurgi ved behov. En slik studie, den såkalte NEEDS- studien, ble nylig startet. Ved operabel PEK i spiserør eller GEJ randomiseres pasienter til enten neoadjuvant radiokjemoterapi + kirurgi eller definitiv radiokjemoterapi etterfulgt av tett oppfølging + senere kurativ kirurgi ved tilbakefall.
Adenokarsinom (AK)
På bakgrunn av metaanalyser og større prospektivt, randomisert kontrollerte studier, kan både perioperativ kjemoterapi som det gis ved kreft i magesekk og neoadjuvant radiokjemoterapi beskrevet over anses som standardbehandling ved lokalavansert AK i spiserør inklusive GEJ (Allum et al., 2009; Ronellenfitsch, Schwarzbach, Hofheinz, Kienle, Kieser, Slanger, & Jensen, 2013; Sjoquist et al., 2011).
Den omdiskuterte MAGIC studien etablerte perioperativ kjemoterapi ved AK i magesekk, distale spiserør og cardia. Denne påviste en bedring i 5-års overlevelse fra 23 % til 36 % for pasienter med resektabel tumor i stadium II og III som ble behandlet med 6 kurer (3 pre- og 3 postoperativt) perioperativ ECF (epirubicin, cisplatin og kontinuerlig infusjon med 5-FU) sammenlignet med kirurgi alene. Studien inkluderte totalt 503 pasienter med AK i distale spiserør (11 %), cardia (15 %) eller magesekk (74 %). Det var imidlertid kun 55 % som startet den postoperative behandlingen og 42 % som fullførte alle 6 kurene (Cunningham et al., 2006).
Senere har den tyske FLOT4-AIO studien vist at et trippelregime med oxaliplatin, 5FU og docetaxel perioperativt (4 kurer før og 4 kurer etter kirurgi) ga bedre median- og 3-års overlevelse enn perioperativ ECF/ECX ved resektabel AK i magesekk og GEJ (S.-E. Al-Batran et al., 2019). Majoriteten hadde cT3–4cN+, og 56 % hadde svulster i den gastroøsofageale overgang. Den viste en median overlevelse på 35 mnd. vs. 50 mnd. (HR 0,77) og 3- års overlevelse på 48 % vs. 57 % til fordel for FLOT (S.-E. Al-Batran et al., 2017). Dette regimet har nå erstattet perioperativ ECX/EOX ved stadium II–III AK i magesekk og cardia.
Neoadjuvant radiokjemoterapi vs. perioperativ kjemoterapi ved AK
Om neoadjuvant radiokjemoterapi etter CROSS regimet eller perioperativ kjemoterapi etter FLOT eller MAGIC regime er å foretrekke, er ikke avklart Det pågår studier som undersøker dette. ESOPEC studien sammenligner perioperativ kjemoterapi etter FLOT protokoll med neoadjuvant radiokjemoterapi etter CROSS protokoll ved multimodal behandling av ikke-metastatisk resektabel AK i spiserør og GEJ (Hoeppner et al., 2016). NeoAEGIS studien ble presentert på ASCO i 2021. Studien (Reynolds et al., 2021) randomiserte pasienter med AK i GEJ til enten neoadjuvant radiokjemoterapi («CROSS») eller til MAGIC eller FLOT type perioperativ kjemoterapi. 377 pasienter ble randomisert til en av behandlingene. Studien ble avsluttet etter at data og sikkerhets komité hadde gjennomgått studien og ikke funnet noen forskjell i resultatene for de to forskjellige behandlingsarmene.
Neoadjuvant radiokjemoterapi vs. neoadjuvant kjemoterapi
Det er heller ikke endelig avklart om neoadjuvant radiokjemoterapi eller neoadjuvant kjemoterapi er å foretrekke. Den skandinaviske NEORES 1 studien randomiserte 181 pasienter med resektabel spiserørskreft inklusive Siewert type 1 og 2 til enten neoadjuvant kjemoterapi eller neoadjuvant radiokjemoterapi etterfulgt av kirurgi. Kjemoterapi besto av 3 CiFu kurer, og stråleterapien besto av 40 Gy fordelt på 20 fraksjoner gitt konkomitant med de to siste CiFu kurene. 27 % av pasientene hadde PEK og 73 % hadde AK. Primært endepunkt var komplett patologisk respons (cPR). Til tross for at man fant en signifikant høyere andel med pCR (9 vs. 28 %), høyere R0 reseksjonsrate og lavere andel med lymfeknutespredning til fordel for neoadjuvant radiokjemoterapi, fant man ingen forskjell i 3-års og 5 års overlevelse (47 vs. 48 %) (Klevebro et al., 2016; von Döbeln et al., 2019).
Ytterlige to relativt små, men randomiserte studier har heller ikke klart å vise noen forskjell mellom neoadjuvant kjemoterapi vs. neoadjuvant radiokjemoterapi (Burmeister et al., 2011; Stahl et al., 2009). Alle tre studiene inkluderte overveiende AK i spiserøret/GEJ.
Perioperativ kjemoterapi vs. neoadjuvant kjemoterapi + adjuvant radiokjemoterapi
I den nederlandske CRITICS studien sammenlignes perioperativ kjemoterapi etter MAGIC-regime mot samme neoadjuvante kjemoterapi, men adjuvant radioterapi med konkomitant cisplatin og capecitabin ved resektabel stadium Ib – III magesekk- og overgangskreft. Kirurgien var standardisert og innebar modifisert D2 lymfeknutedisseksjon. Denne viste ingen tilleggs gevinst av postoperativ radiokjemoterapi hos pasienter som hadde fått neoadjuvant kjemoterapi (Cats et al., 2018).
Perioperativ kjemoterapi vs. neoadjuvant kjemoterapi + radiokjemoterapi + adjuvant kjemoterapi
I TOPGEAR studien sammenlignes perioperativ kjemoterapi etter MAGIC-regime mot neoadjuvant radiokjemoterapi og adjuvant kjemoterapi ved resektabel stadium Ib – III (unntatt T2N0) magesekk- og overgangskreft (Leong et al., 2015). 27 % av pasientene hadde kreft i GEJ. En interim-analyse har vist lik hematologisk toksisitet i begge behandlingsarmene, litt mer øsofagitt i stråleterapiarmen, men ellers ingen forskjell i toksisitet. Endelige resultater avventes.
Adjuvant immunterapi
I den nylig publiserte CheckMate 577 studien (Kelly et al., 2021), en global, randomisert, dobbeltblind, placebokontrollert fase 3-studie ble nivolumab (PD-1 hemmer) undersøkt som adjuvant behandling til pasienter med kreft i spiserør eller gastroøsofageal overgang etter neoadjuvant radiokjemoterapi, og som hadde gjenværende tumorvev i resektatet. Deltagere ble randomisert 2:1 til enten nivolumab (240 mg hver 2. uke i 16 uker, etterfulgt av nivolumab 480 mg hver 4. uke) eller placebo. Behandlingsvarighet var maksimalt 1 år. Blant de 532 pasientene som fikk nivolumab, var median sykdomsfri overlevelse (PFS) 22,4 måneder vs. 11,0 måneder blant de 262 pasientene som mottok placebo (HR residiv eller død, 0,69, P <0,001).
Adjuvant behandling ved R1
For pasienter som er operert med ufrie reseksjonsmarginer, bør indikasjon for adjuvant behandling vurderes i multidisplinært møte (Cats et al., 2018).
Anbefalinger
T1 N0: kirurgi direkte
For lokalavansert sykdom (cT2–4 eller cN1–3 M0) anbefales neoadjuvant radiokjemoterapi.
For adenokarsinomer er perioperativ kjemoterapi med FLOT-regimet et alternativ.
For pasienter operert for kreft i spiserør eller gastroøsofageale overgang, etter neoadjuvant radiokjemoterapi og som ikke har oppnådd pCR i resektatet, kan adjuvant behandling i inntil 1 år med PD-1 hemmeren nivolumab vurderes.
Definitiv radiokjemoterapi
Definitiv radiokjemoterapi kan tilbys til pasienter med lokal ikke-resektabel spiserørskreft, medisinsk inoperable pasienter og til pasienter som ikke ønsker operasjon. Definitiv radiokjemoterapi gis i kurativ hensikt, og kan tilbys pasienter man antar kan tolerere denne behandlingen.
I en studie fra USA (RTOG 85-01) viste man at cytostatika kombinert med strålebehandling bedret overlevelsen sammenlignet med strålebehandling alene (5 års ovelevelse var 26 % ved radiokjemoterapi vs. 0 % ved kun radioterapi) (Cooper et al., 1999). I en oppfølgerstudie (INT 0123) studerte man effekten av å øke stråledosen, men man fant at økning av stråledosen til 64,8 Gy ikke bedret regional kontroll eller overlevelse sammenlignet med samme type cytostatika kombinert med strålebehandling til 50,4 Gy (Minsky et al., 2002). I begge disse studiene hadde >80 % av pasientene PEK. Siden sistnevnte studie ble publisert i 2002 har strålebehandlingsteknikken bedret seg. I en nylig randomisert studie, der pasienter med PEK som fikk definitiv radiokjemoterapi med intensitets modulert radioterapi (IMRT) og kjemoterapi, fant man ingen gevinst i lokalt tilbakefall, progresjonsfri overlevelse (PFS) eller total overlevelse (OS) ved å øke stråledose fra 50 Gy til 60 Gy (Y. J. Xu et al., 2020).
I en retrospektiv studie fra MD Anderson sykehuset i USA fant man at 50 % av pasientene som fikk definitiv strålebehandling til 50,4 Gy samtidig med cytostatika utviklet lokaltregionalt tilbakefall, 90 % av disse tilbakefallene var innenfor GTV, 48 % av pasientene utviklet fjernmetastaser, mens 31 % ikke fikk påvist tilbakefall i observasjonsperioden. Behandlingssvikt innenfor GTV var assosiert med T3 og T4 sykdom og tumordiameter over 8 cm (Welsh et al., 2012). Flere andre retrospektive studier har også vist at en høy andel av pasientene som har fått cytostatika kombinert med strålebehandling til 50 Gy, og ikke blir operert med reseksjon av tumor, har terapisvikt og progresjon av kreftsykdom innenfor tidligere gitte strålefelt.
Det er også vist at det foreligger en korrelasjon mellom høyere stråledoser og bedret lokoregional kontoll, men at dose respons kurven flater av i de høyest undersøkte dosenivåene (Z. Zhang et al., 2005). I den nylig publiserte ARTDECO studien, ble 260 pasienter med inoperabel lokalavansert spiserørskreft (62 % PEK og 38 % AK) randomisert mellom standard dose 50,4 Gy mot tumor og regionale lymfeknuter (SD) og høy dose på 61,6 Gy mot primærtumor (HD) (Hulshof et al., 2021). Kjemoterapien besto av ukentlig karboplatin (AUC2) og paklitaksel (50mg/m2) i 6 uker. Tre års lokal progresjonfri overlevelse (LPFS) var 71 % i SD-armen og 73 % i HD-armen. LPFS for PEK og AK var henholdsvis 75 % mot 79 % og 61 % mot 61 % for SD og HD (ikke signifikant). Tre års lokalregional progresjonsfri overlevelse var henholdsvis 52 % og 59 % for SD- og HD-armene (P
p = 0,08). Det var ingen forskjell i 3-års OS som var 42 % (SD) versus 39 % (HD). Studien konkluderte med at doseekalert strålebehandling mot primærtumor ikke gir noen bedret lokal kontroll eller overlevelsesgevinst, kun økt toksisitet.
Kjemoterapien som ble benyttet i RTOG og INT studiene var Cisplatin/5-FU (totalt 4 kurer), 2 konkomitante (uke 1 og 5) og 2 adjuvante etter strålebehandling (uke 8 og 11). I en fransk studie PRODIGE5/ACCORD17 randomiserte man mellom 6 kurer FOLFOX hvorav 3 ble gitt konkomitant, mot kjemoterapi med Cisplatin/5-FU, og man fant ingen signifikant forskjell i PFS (hhv. 9,7 mnd. vs. 9,4 mnd.), median OS (hhv. 20,2 mnd. vs. 17,5 mnd.) eller 3 års overlevelse (19,9 mnd. vs. 26,9 mnd.), og de konkluderte med at FOLFOX kurer kan være et like godt alternativ som Cisplatin/5-FU kurer. I denne studien hadde 85 % av pasientene PEK (Thierry Conroy et al., 2014).
Flere sentra benytter forlengede CROSS regime med 6 ukeskurer Carboplatin/Palitaxel under definitiv radiokjemoterapi og strålebehandlinge til 50,4 Gy. Det finnes ingen direkte prospektiv sammenlikning av dette radiokjemoterapi-regimet med de andre regimene (2Gy x 25 med CiFU eller FOLFOX), men 3-års overlevelsen i ARTDECO studien på om lag 40 % tyder på at forlenget CROSS radiokjemoterapi kan være et godt alternativ (Thierry Conroy et al., 2014; Hulshof et al., 2021; M. A. van Ruler et al., 2017). Dette er også undersøkt i en rekke retrospektive studier (Münch et al., 2018; Owens et al., 2020; Qu, Biagi, Hopman, & Mahmud, 2017; Steber et al., 2021; M. A. van Ruler et al., 2017).
Pasienter med AK i spiserøret har også vært inkluderte i studiene over, men ofte i mindretall, slik at nytten for definitiv radiokjemoterapi hos disse, sammenliknet med kjemoterapi alene, er noe mer uklar.
Hvordan strålefeltene bør utformes for at pasientene skal ha størst mulig nytte om minst mulig bivirkninger av behandlinger blir også undersøkt i pågående studier.
Cervikal spiserørskreft
Cervikal spiserørskreft er en sjelden undergruppe av spiserørskreft, og er nesten utelukkende PEK. For disse pasientene anbefales vanligvis radikal/definitiv radiokjemoterapi. Flere mindre studier viser at strålebehandling til høyere stråledoser enn 50 Gy gir god lokal kontroll. I en retrospektiv studie fra Tyskland der 56 pasienter med cervikal spiserørskreft i stadium II og III fikk strålebehandling til median dose på 60 Gy (50–70 Gy) konkomitant med cytostatika var 2 og 5 års overlevelse på henholdsvis 35 % og 29 %, og lokalt eller regionalt tilbakefall ble påvist hos 44 % av pasientene (Gkika et al., 2014). I en studie fra Australia der 34 pasienter med cervikal spiserørskreft i stadium I-III fikk strålebehandling til gjennomsnittlig dose på 61,2 Gy (50,4–65 Gy) kombinert med cytostatika var 5 års overlevelse på 55 % (Burmeister, Dickie, Smithers, Hodge, & Morton, 2000). I flere mindre retrospektive studier fra Asia rapporterer man at definitiv radiokjemoterapi til median stråledose over 60 Gy gir 5 års overlevelse som er sammenlignbar med historiske kontrollmaterialer hvor pasientene har fått kirurgisk behandling.
I en studie fra Toronto i Canada kunne man ikke påvise at 42 pasienter som fikk definitiv radiokjemoterapi til 70 Gy hadde bedret lokal kontroll eller bedret overlevelse sammenlignet med en historisk kontrollgruppe på 29 pasienter som hadde fått definitiv radiokjemoterapi til 54 Gy. Andelen av pasienter med mer avansert stadium var imidlertid høyere blant pasientene som hadde fått strålebehandling til 70 Gy (S. H. Huang et al., 2008). I en prospektiv fase II studie for operabel cervikal spiserørskreft, så man på effekt av strålebehandling til 60 Gy sammen med 2 konkomitante Cisplatin/5-FU kurer gitt med 4 ukers mellomrom (Zenda et al., 2016). Man fant komplett klinisk respons hos 73 % av pasientene, og 3-års overlevelse var 66,5 %.
Kurdefinisjoner
Carboplatin/Paclitaxel: Carboplatin (AUC2) dag 1 og Paclitaxel 50 mg/m² dag 1. Gis ukentlig.
CiFu: Cisplatin 75 mg/m²dag 1, 5-FU 750–1000 mg/m²/døgn dag 1–4. Gis hver 3. uke, men 4 uker mellom 2 første kurer under strålebehandlingen.
Før behandling skal hørsel og nyrefunksjon (kreatinin clearence) kontrolleres. Ved sviktende nyrefunksjon eller hørsel kan cisplatin skiftes ut med oksaliplatin eller karboplatin (T. Conroy et al., 2010; van Meerten et al., 2006).
Bruk av granulocytt-kolonistimulerende faktor (G-CSF) må overveies ved alvorlig neutropeni og episoder med febril neutropeni.
OxFu: Oxaliplatin 130 mg/m² dag 1, 5-FU 1000 mg/m²/døgn dag 1–4. Gis hver 3. uke, men 4 uker mellom 2 første kurer under strålebehandlingen.
FOLFOX: Oxaliplatin 85 mg/m² dag 1, 5-FU bolus 400 mg/m² dag 1 etterfulgt av 5-FU 800 mg/m²/døgn dag som kontinuerlig infusjon i 48 timer. Gis hver 2. uke.
FLOT: docetaksel 50 mg/m2 D1, oksaliplatin 85 mg/m2 D1, kasliumfolinat 200 mg/m D1 og 5FU 2600 mg/m2 D1–2
For administrasjon av kontinuerlig cytostatikainfusjon, spesielt ved bruk av infusjonspumpe, trenger pasienten sentralt venekateter. Pre- og posthydrering i forbindelse med cisplatin er avgjørende for å få pasienten gjennom behandlingen uten nyresvikt. På grunn av at mange pasienter har dysfagi, ofte med stort vekttap, anbefales at innleggelse av perkutan gastrostomi (PEG) eller nasogastrisk sonde vurderes for å optimalisere ernæringstilførselen i behandlingstiden. Før innleggelse av en eventuell gastrostomi må en konsultere kirurg for å avklare om dette vil komme i konflikt med mulig fremtidig kirurgisk behandling. Optimalisering av ernæring anbefales i samråd med ernæringsfysiolog.
Strålebehandling: Målvolum er primærtumor og regionale lymfeknutestasjoner. Makroskopisk tumor (GTV) bør få en totaldose på 50 Gy, mikroskopisk sykdom 46 Gy. Prinsipielt har man ved tumor lokalisert kranialt for carina inkludert fossae supraclaviculares og ved tumor kaudalt for carina inkludert cøliacusområdet. For detaljer i strålebehandlingsopplegget vises til «Faglige anbefalinger ved stråleterapi av spiserørskreft» (Faglige anbefalinger for strålebehandling ved øsofaguscancer, 2009).
Selv om det ikke foreligger dokumentasjon fra randomiserte studier anbefales det at man ut fra pasientens forventede toleranse vurderer strålebehandling til totaldose 60–66 Gy for pasienter med svulster i cervikale del av spiserøret. For svulster i distale del av spiserøret, hvor strålefeltet som oftest inkluderer proksimale del av magesekken og hvor det foreligger regionale lymfeknutemetastaser langs curvatura minor og ved truncus cøliacus, anbefales at man er tilbakeholden med å gå høyere enn 50,4 Gy i stråledose.
Anbefaling
Definitiv radiokjemoterapi kan tilbys pasienter med lokal ikke resektabel spiserørskreft, medisinsk inoperable pasienter som vurderes å tolerere behandlingen, og pasienter som ikke ønsker operasjon (evidensgrad A).
Ved svulster i cervikale spiserøret kan man ut ifra en individuell vurdering av pasientens toleranse vurdere strålebehandling til totaldose 60–66 Gy. Ved svulster i distale del av spiserøret og gastroøsofageal overgang anbefales det ikke å overstige totaldose på 50,4 Gy (evidensgrad A).
Ernæring
Sist faglig oppdatert: 28.06.2022
Pre- og postoperativ ernæring
Preoperativ ernæring er indisert for pasienter med vekttap >10–15 % siste 6 måneder, kroppsmasseindeks < 18 kg/m2 og/eller albumin < 30 g/l. Ernæringssituasjonen hos underernærte pasienter bør evalueres i samarbeid med klinisk ernæringsfysiolog spesielt med tanke på å forebygge reernæringssyndrom.
Hvis mulig bør ernæringen gis enteralt (Weimann et al., 2006) via sonde til magesekk eller duodenum, alternativt parenteralt (Braga et al., 2009) via sentralt venekateter. Behandlingstiden bør være minst 7–10 døgn. Hvis pasienten er i stand til å innta tilstrekkelig peroral tilførsel av næringsrike drikker, kan dette erstatte behovet for enteral og/eller parenteral ernæring.
Postoperativt skal pasienter som ikke er underernært, så snart som mulig begynne med peroralt inntak av næringsstoffer. Ved inadekvat næringsinntak i 5–7 dager skal ernæringen suppleres helst enteralt, alternativt parenteralt. Pasienter som er underernært og ikke har fått preoperativ adekvat ernæring, skal begynne med enteral ernæring, eventuelt supplert med partiell parenteral ernæring, fra første postoperative dag. Den enterale ernæringen kan tilføres via sonde plassert forbi anastomosen i thorax (øsofagogastrostomien), nasogastrisk eller nasoenteralt. Dessuten kan jejunumkateter plasseres peroperativt.
Ernæring ved onkologisk behandling
Ved kombinert stråle- og cytostatikabehandling anbefales ekstra enteral ernæring med næringsdrikker og eventuelt ernæring via sonde/PEG (Bozzetti, 2010).
Det kan være hensiktsmessig å henvise til klinisk ernæringsfysiolog ved oppstart onkologisk behandling, spesielt ved radiokjemoterapi.
For generelle råd om ernæring vises til «Nasjonale faglige retningslinjer for forebygging og behandling av underernæring» fra 2009 (Nasjonal faglig retningslinje for forebygging og behandling av underernæring, 2009).
Pasienter som er stentet, kan (delvis) ernæres peroralt. Ved utilstrekkelig ernæring peroralt kan re-stenting eller endoskopisk eller kirurgisk innleggelse av gastrostomikateter være alternativer. Komplikasjoner til gastrostomikateter er særlig dislokasjon og sårinfeksjon.
Oppfølging og kontroll etter avsluttet kurativ behandling
Sist faglig oppdatert: 28.06.2022
Pasienter med invasiv kreft behandlet med radikal kirurgi, bør kontrolleres med hensyn til spisefunksjon og ernæring det første året. 10–20 % av opererte pasienter utvikler anastomosestriktur, og det kan her være god effekt av repeterte endoskopiske dilatasjoner.
Pasienter som behandles med radio- og/eller kjemoterapi bør følges med tanke på senvirkninger, spesielt hjerte- og lungefunksjon. Senere kontroller er aktuelle for registrering og oppfølging av senvirkninger etter strålebehandling ved kombinert behandling. Pasienter som er behandlet med definitiv radiokjemoterapi og som kan være aktuelle for reseksjon av spiserør ved residiv bør følges med regelmessige kontroller.
Det er ingen evidens for at regelmessig oppfølging forbedrer overlevelsen (Oesophageal and gastric cancers: diagnosis and assessment, 2001), men det er av betydning å registrere behandlingsresultatet i det nasjonale spiserørskreft-registeret. Retrospektive undersøkelser har vist at påvisning av residiv hos den asymptomatiske pasient ikke resulterte i forlenget overlevelse sammenlignet med overlevelsen hos pasienter som ble fanget opp ved symptomatisk residiv. Ved symptomatisk residiv undersøkes pasienten adekvat for lokoregionalt residiv og/eller fjernmetastaser (endoskopi, CT). Palliative tiltak kan være aktuelt ved påvist residiv, og retter seg i første hånd mot å holde spiserøret åpent (se senere). En skandinavisk randomisert studie på eventuell overlevelsesgevinst ved systmatisk kontroll etter gastroøsofageal reseksjon er under utarbeidelse (Sarong studien). Det er vist at pasienter som gjennomgår øsofagusreseksjon har redusert livskvalitet sammenlignet med normalbefolkningen i inntil 20 år etter operasjon (Ref: Boshier.AoS.2020).
Anbefaling
Kontroll etter kurativ behandling er viktig for å sikre adekvat ernæringsfunksjon (evidensgrad D).
Ved komplett respons hos pasienter som har gjennomgått definitiv radiokjemoterapi kan utvalgte pasienter være aktuelle for salvage kirurgi ved residiv.
Pasienter som har gjennomgått endoskopisk behandling for dysplasi eller kreft i tidlig stadium skal kontrolleres ved behandlende avdeling (evidensgrad D)
Etter endoskopisk behandling bør kontroll med gastroskopi foretas hver 3. måned i ett år, deretter årlig (evidensgrad D).
Disse anbefalingene er diskutert i fagrådet for kvalitetsregisteret for kreft i spiserør og magesekk og de vil også diskuteres før neste revisjon av handlingsprogrammet. Det er per i dag, vanskelig å anbefale noe annet enn det som nå står (og som har vært anbefalt tidligere).
Behandling av metas-taserende sykdom/ Livsforlengende og palliativ behandling
Behandling av lokale symptomer
Sist faglig oppdatert: 28.06.2022
De fleste pasienter som får diagnosen spiserørskreft, har en sykdom i avansert stadium med plagsomme lokale symptomer i form av dysfagi, smerter og slimdannelse. Det er spesielt viktig for pasientene å klare å svelge sitt eget spytt, spise selv og fortrinnsvis oppnå en normal ernæringsfunksjon. Målet ved palliativ behandling er å lindre disse plagene. Se eget handlingsprogram for palliasjon for utfyllende informasjon.
Det foreligger flere metoder for å lindre symptomer:
Ekstern strålebehandling med ulike fraksjoneringsregimer brukes ofte for lindring av plager fra lokalavansert spiserørskreft. Hvilket fraksjoneringsregime som er å foretrekke er ikke kjent. En retrospektiv studie fra Nederland publisert i 2019 sammenlignet ulike fraksjoneringsregimer (4 Gy x 5 vs. 3 Gy x 10 vs. 3 Gy x 13). Dette var pasienter som ikke hadde fått selvekspanderende metallstent (SEMS). Man fant en lindring av symptomer hos 72 % av pasientene (bedring av dysfagi, smertelindring eller reduksjon av tumorblødning), og det var ingen forskjell mellom gruppene. Man fant at høyere stråledose (30 – 39 Gy) var assosiert med lengre tid til behov for ny intervensjon. Videre fant man at redusert allmenntilstand (Karnofsky performance score 60-80 vs. 90-100), sykdomsresidiv og tilstedeværelse av fjernspredning var signifikant assosiert med redusert overlevelse (Walterbos et al., 2019).
Selvekspanderende metallstent (SEMS) kan benyttes for palliasjon av dysfagi og ved øsofagotrakeale fistler og gir ofte god og rask lindring (M. Y. Homs et al., 2005). En dekket stent kan tette en øsofageotrakeal fistel og derved forebygge aspirasjonspneumoni. Det finnes ulike typer og lengder av stenter. Det er imidlertid en grense (1–2 cm nedenfor øvre sfinkter) for hvor langt proksimalt stenten kan plasseres uten å forårsake smerter og aspirasjonsfare. Prosedyren kan utføres i lett sedasjon, røntgen- og endoskopiassistert. De aller fleste kjenner ubehag/smerte retrosternalt de første to døgn etter stentnedleggelse. Andre tidlige komplikasjoner (første uken) er perforasjon, blødning og dislokasjon av stenten. Senere komplikasjoner er overvekst av tumorvev, tilstopping av stenten med matbiter og migrasjon av stenten (oftest distalt) (Wenger, Luo, Lundell, & Lagergren, 2005). Det er mulig å legge ned stent flere ganger, med eller uten fjerning av den gamle.
I en metaanalyse fra 2010 som inkluderte 1027 pasienter fra 16 randomiserte, kontrollerte studier var konklusjonen at anleggelse av selvekspanderende stent ga mindre behov for reintervensjoner enn andre lokoregionale behandlingsmodaliteter. Det ble videre ikke påvist noen fordel ved å bruke stenter med antirefluksmekanisme/-ventil der stentene legges over den gastroøsofageale overgang (Sgourakis et al., 2010).
En metaanalyse publisert i 2018 sammenlignet bl.a. SEMS i kombinasjon med strålebehandling eller kjemoterapi, versus SEMS alene. Man fant ingen forskjell i dysfagi på kort sikt, men blant de som levde lengre enn 3 mnd. var det en signifikant forskjell i dysfagi til fordel for kombinasjonsbehandling. Man fant også en signifikant bedring i overlevelse og livskvalitet til fordel for kombinasjon (Lai et al., 2018).
Brakyterapi: Kan gis alene, fraksjonsdose 8 Gy gitt én gang i uken i alt tre ganger, eller i kombinasjon med stent. I en nederlandsk randomisert studie fant man at brakyterapi gav bedre langtidsremisjon av dysfagi enn stent og med mindre komplikasjoner (M. Y. V. Homs et al., 2004). Brakyterapi kan også gis i kombinasjon med ekstern strålebehandling (Bergquist et al., 2005), 8 Gy gitt én gang i uken to ganger etter ekstern 30 Gy. Brakyterapi er ikke tilgjengelig i Norge for denne pasientgruppen per i dag,
Cytostatika: Ved tumor bare i spiserøret og symptomer derfra, anbefales lokal behandling. Cytostatika gitt for systemisk sykdom har ofte god effekt også lokalt.
Dilatasjon: Det er ikke indikasjon for dilatasjon som eneste behandling.
Endoskopisk koagulasjon, laser eller argon plasma koagulasjon (APC), kan være godt egnet ved behandling av polypøse svulster og ved blødning fra tumor.
Bypass-kirurgi og palliativ reseksjon: Ved perforasjon vurderes en sjelden gang palliativ reseksjon eller bypass. Det finnes knapt indikasjon for elektiv reseksjon av spiserør ved påviste fjernmetastaser eller innvekst i naboorganer.
Ernærningssonde eller perkutan endoskopisk gastrostomi (PEG): Ved ernæringsproblemer bør pasienten få hjelp til enteral ernæring. Parenteral ernæring bør unngås ved avansert sykdom. Det vises til kapittel Ernæring.
Behandling ved metastatisk og utbredt sykdom
Sist faglig oppdatert: 28.06.2022
Systemisk behandling (cytostatika og immunterapi)
Spiserørskreft med fjernmetastaser er ikke mulig å helbrede, og intensjonen for all behandling er derfor palliativ. Hensikten med cytostatikabehandling er å stabilisere sykdommen, lindre plager, forlenge symptomfattig periode og helst også øke levetiden. I en Cochrane-review er det vist at kombinasjonskjemoterapi bedrer både livskvalitet og overlevelse framfor monoterapi, men har økt toksisitet (Wagner et al., 2017). Det er rapportert responsrater på 17–51 % og median overlevelse på 6–11 måneder. Det er økende evidens for nytten av immunterapi både alene og i kombinasjon med kjemoterapi ved kreft i spiserør, magesekk og GEJ, både i første og senere linjer (se under), og forventes vil bli brukt i økende grad framover.
Tidlig integrasjon av palliativt team?
Fordelen med tidlig tverrfaglig støttende behandling ble vist i en kinesisk studie der 328 pasienter med tidligere ubehandlet metastatisk kreft i spiserør eller magesekk ble randomisert til tidlig tverrfaglig omsorg med fokus på ernæring og psykisk helse integrert i standard onkologisk behandling eller til standard behandling. Intervensjonsgruppen fikk en tverrfaglig støttende konsultasjon innen 14 dager etter oppstart kjemoterapi, og deretter en oppfølgingskonsultasjon hver tredje uke. Median OS var signifikant bedre i tidlig intervensjonsgruppe (14,8 mot 11,9, HR 0,68, 95 % KI 0,51-0,9) (Lu et al., 2021). Om disse resultatene er overførbare til norske forhold, vites ikke.
Plateepitelkarsinom (PEK) eller adenokarsinom (AK)
Det kan skilles mellom behandling av AK og PEK i spiserøret. For PEK har kombinasjonsbehandling med 5FU og cisplatin vist økt responsrate sammenlignet med monoterapi med 5FU, men overlevelsesgevinst er ikke dokumentert (Levard et al., 1998). Imidlertid er det ved flere større studier fra slutten av 90-tallet inkludert både AK og PEK, og man har da ikke sett sikre forskjeller i effekt mellom de ulike histologiske subgruppene (Ross et al., 2002; Webb et al., 1997). Praksis er derfor at man (ved valg av kjemoterapi) ikke nødvendigvis skiller mellom behandling av PEK og AK i spiserøret. Mange av studiene består av en blanding av pasienter med spiserørs-, gastroøsofageal overgangs- og magesekkreft, og anbefalingene for behandling av kreft i magesekken og spiserøret er derfor i mange tilfeller svært lik.
Med utvikling av molekylært rettet behandling og immunterapi har behovet for å skille mellom AK og PEK blitt mer tydelig, samt viktighet av å gjøre ulike molekylære tester. Eksempelvis har HER2-rettet (trastuzumab) og VEGF-rettet (ramucirumab) behandling kun vist effekt ved behandling av (undergrupper av) AK. PD-L1 uttrykk og MSI-status kan ha betydning for effekt av immunterapi.
Vi vil derfor anbefale at det testes for HER-2 (AK), MSI/MMR, PD-L1 der det angis CPS ved AK og at det angis både CPS og TPS ved PEK, og eventuelt for EBV når det planlegges for systemisk tumorrettet behandling.
1. linje systemisk behandling
1. linje kjemoterapi ved AK PD-L1 CPS < 5 eller dersom immunterapi er kontraindisert eller ikke tilgjengelig
Det er vist overlevelsesgevinst ved cellegiftbehandling både i 1.- og 2.-linje sammenlignet med «Best supportive care» (BSC). En Cochrane-review fra 2017 viste at cellegiftbehandling gir en forlenget overlevelse på 6,7 mnd sammenlignet med BSC (Wagner et al., 2017). Flere ulike cytostatikagrupper er vist å ha effekt, og ved valg av terapi er det derfor viktig å se både på selve behandingseffekten og på bivirkningsprofilen.
Trestoffs-kombinasjonen av cisplatin, 5FU og epirubicin (ECF) var tidligere ansett som standard førstelinjes behandling i Europa og Norge. REAL2 studien fra 2008 viste at oxaliplatin i stedet for cisplatin og capecitabine i stedet for iv 5FU (EOX) var like effektivt i kombinasjon med epirubicin. OS i EOX-gruppen i denne studien var 11,2 måneder (Cunningham et al., 2008).
Docetaxel i kombinasjon med cisplatin og 5FU (DCF) har vist en noe bedre OS enn CF alene (OS 9,2 mnd vs 8,6 mnd), men høyere toxisitet i taxangruppen gjør denne kombinasjonen mindre aktuell (Van Cutsem et al., 2006). En senere studie med modifisert DCF har antydet at man kan oppnå minst like gode resultater ved å doseredusere, og samtidig oppnå langt lavere toksisitet (Shah et al., 2015).
FLOT-regimet (5FU, oxaliplatin og docetaxel) har i to mindre studier vist lovende resultater med PFS på hhv 5,1 og 7,7 md, og OS på 11 og 14,6 md (S. E. Al-Batran et al., 2008; Van Cutsem et al., 2015).
Trestoffs-regimer har en ikke ubetydelig toxisitet, og det er gjort flere studier hvor man har sammenlignet disse med kombinasjoner av to stoffer i 1.-linjes behandling. Irinotecan i kombinasjon med 5-FU er vist å ha sammenlignbar PFS og OS med trestoffs-regimet ECX (epirubicin, cisplatin og capecitabin) (Guimbaud et al., 2014). Man har også sett lik effekt av oxaliplatin i kombinasjon med 5-FU sammenlignet med ECF (Enzinger et al., 2016). Disse resultatene har også gjort at nytten av epirubicin har blitt diskutert. En metaanalyse fra 2010 viste signifikant overlevelsesgevinst ved tillegg av antracycliner til cisplatin og 5-FU (Wagner et al., 2017), men en senere meta-analyse konkluderer med manglende tilleggsgevinst av antracyclin (Ter Veer et al., 2016). I tillegg konkluderer denne meta-analysen med at et fluoropyrimidinholdig tostoffs-regime med oxaliplatin, irinotecan eller et taxan er å foretrekke framfor et cisplatinholdig tostoffs-regime, et antracyclinholdig trestoffs-regime eller et TCF regime (taxan, cisplatin, 5FU) i første linjes behandling ved avansert gastroøsofageal kreft. Det mest lovende trestoffs-regimet var vurdert å være kombinasjon av fluoropyrimidin, oxaliplatin og et taxan.
Trestoffs-kombinasjon med ECX/EOX i palliativ setting er i dag forlatt.
Trastuzumab
ToGa-studien, en randomisert fase III-studie, undersøkte effekten av trastuzumab i kombinasjon med kjemoterapi i 1. linje ved HER2-positive AK i GEJ og magesekk. Det ble påvist en signifikant bedret median OS i favør av trastuzumab-gruppen (13,8 vs 11.1 mnd) (Bang et al., 2010). Pasienter med høyest ekspresjon av HER2 (IHC 2+ og FISH+, eller IHC 3+) hadde størst gevinst med median overlevelse på 16 mnd.(Bang et al., 2010)
VEGF- og EGFR- hemmere
Både bevacizumab, cetuximab og panitumumab har blitt testet ut i studier uten å kunne demonstrere noen tilleggseffekt til kjemoterapi i 1. linje. (156-158).
Immunterapi i 1. linje
Det er dokumentert gode resultater med immunterapi ved bruk av antistoffene pembrolizumab eller nivolumab rettet mot PD-1 ved metastaserende AK i magesekk, GEJ og spiserør og PEK i spiserør.
Adenokarsinom (AK)
Det er utført flere større randomiserte studier som undersøker PD-1 hemmer i førstelinjes behandling ved kreft i spiserør, GEJ og magesekk. KEYNOTE-062 (Shitara, Van Cutsem, et al., 2020) var en tre-armet randomisert fase III-studie som randomiserte 1:1:1 til enten pembrolizumab monoterapi, pembrolizumab + kjemoterapi eller til kjemoterapi alene med cisplatin + 5FU/ capecitabine i førstelinjes behandling av avansert/metastatisk AK (Her-2 neg, CPS≥1) i magesekk eller GEJ. Primære endepunkt var totaloverlevelse og progresjonsfri overlevelse ved CPS ≥1. Man kunne ikke vise noen bedret overlevelse sammenliknet med kjemoterapi alene. Studien bestod av 58 % vestlige pasienter, 24 % asiatiske og 17 % fra andre regioner. Studien er eneste fase III studie så langt med bruk av PD-1 hemmer som monoterapi i førstelinjes behandling, og overlevelsesdata illustrerer såkalt «kryssende Kaplan-Meier kurver» for pembrolizumab monoterapi armen sammeliknet med kjemoterapi. Det var lavere responsrater for pembrolizumab sammenliknet med kjemoterapi i første linje. Objektiv responsrate var hhv. 15 % (pembrolizumab monoterapi), 49 % (pembrolizumab + kjemoterapi) og 37 % (kjemoterapi) hos ITT populasjonen med CPS≥1. Responsen varte mye lengre for pasienter som hadde respons på pembrolizumab (13,7 mnd) sammenliknet med kjemoterapi (6,8 mnd). Selv om studien var negativ mht primært endepunkt, viste subgruppeanalyser bedret overlevelse hos pasienter med mikrosatelittinstabile (MSI) svulster, der median overlevelse ikke var nådd i noen av armene med pembrolizumab, sammenliknet med 8,5 mnd. ved kjemoterapi alene. I en annen subgruppeanalyse ble det funnet forbedret total overlevelse med pembrolizumab sammenlignet med standard kjemoterapi hos pasienter med mikrosatelittstabile (MSS) svulster med PD-L1 CPS≥10 (16,0 mot 10,8 måneder, HR, 0,76) der MSI-høy var eksluderte. Studien konkluderte med at pembrolizumab var non-inferior og med signifikant mindre toksisitet enn kjemoterapi hos pasienter med CPS ≥1 (10,6 mot 11,1 måneder, HR 0,91).
CheckMate-649 (Y. Y. Janjigian et al., 2021), en annen fase III-studie, sammenlignet kombinasjonen nivolumab + kjemoterapi (FOLFOX eller XELOX) versus kjemoterapi alene i 1. linje hos pasienter med avansert HER2-neg. AK i magesekk, GEJ eller spiserør. Pasienter ble inkludert i studien uavhengig av PD-L1 status. Primære endepunkter var OS og PFS hos pasienter med PD-L1 CPS ≥ 5. Nivolumab med kjemoterapi viste en median OS på 14,4 måneder versus 11,1 måneder ved kjemoterapi alene blant de med PD-L1 CPS ≥ 5 (HR 0,71 [0,59-0,86], p <0,0001). Gevinst med nivolumab ble også funnet ved PD-L1 CPS ≥ 1 og i hele kohorten, men den var noe mindre uttalt. Det synes å være spesielt god effekt av kombinasjonen Nivolumab + kjemoterapi ved MSI svulster. I denne studien var median overlevelse for denne pasientgruppen 8,8 mnd. med kjemoterapi alene, mens median overlevelse ikke er nådd med kombinasjonen. Ingen nye alvorlige bivirkninger ble avdekket. Studien konkluderte med at kombinasjonen nivolumab og kjemoterapi kan være en ny standard førstelinjes behandling for pasienter med AK i spiserør/magesekk med CPS≥5.
I en asiatisk fase-2/3 studie, ATTRACTION-4, presentert på ESMO -2020, ble det randomisert 724 pasienter til nivolumab + kjemoterapi eller kun kjemoterapi. PFS var signifikant bedre i nivolumab + kjemoterapi armen (10,45 mnd vs 8,34 mnd; p=0,0007; HR 0,68). Median OS viste ikke signifikat forskjell (17,45 mnd vs 17,15 mnd; p=0,257), men man må ta hensyn til at det var flere pasienter i kjemoterapi armen som mottok immunterapi etter progresjon, noe som er godkjent mange steder i Asia.
Plateepitelkarsinom (PEK) og adenokarsinom (AK)
KEYNOTE-590 (J. M. Sun et al., 2021) var en fase 3 studie som randomiserte 749 pasienter med tidligere ubehandlet lokalavansert eller metastastatisk AK (26 %) eller PEK (74 %) i spiserør (inkl. Siewert 1) uavhengig av PD-L1 status, til enten kombinasjonsbehandling pembrolizumab + kjemoterapi med cisplatin + 5FU eller til placebo + kjemoterapi. Halvparten hadde svulster med PD-L1 CPS ≥ 10, og halvparten av populasjon var ikke-asiatiske. Primære endepunkter var OS i følgende grupper: Spiserørs PEK med PD-L1 CPS ≥ 10, spiserørs PEK, alle med PD-L1 CPS ≥ 10 og alle randomiserte pasienter. Primært endepunkt var også PFS blant spiserørs PEK, alle med PD-L1 CPS ≥ 10 og alle randomiserte.
Median OS og PFS var signifikant lengre med pembro + kjemo vs. placebo + kjemo for alle forhåndsdefinerte grupper. For PEK med CPS ≥ 10 var median OS 13,9 vs. 8,8 mnd., for alle PEK var median OS 12,6 vs. 9,8 mnd, for alle med CPS ≥ 10 var median OS 13,5 vs. 9,4 mnd., og for alle randomiserte var median OS 12,4 vs. 9,8 mnd. To års overlevelsesrate økte i størrelsesorden fra 16 % til 28 % med tillegg av pembrolizumab hos alle randomiserte.
Plateepitelkarsinom (PEK)
CheckMate 648 var en global fase III studie for pasienter med PEK i spiserøret, som randomiserte 1:1:1 til nivolumab 240 mg hver 2. uke + CiFu hver 4. uke; nivolumab 3 mg/kg hver 2. uke + ipilimumab 1 mg/kg hver 6. uke; eller kjemoterapi alene. Primære endepunkt var OS og PFS hos pasienter med såkalt total positiv PD-L1 score (TPS) ≥1 %. Ifølge de første resultatene som ble presentert på ASCO årsmøte i 2021 var også dette en positiv studie, som viste bedret median OSved TPS≥1 % i de eksperimentelle armene, hhv 15,6 mnd med nivolumab + kjemoterapi og 13,7 mnd med nivolumab + ipilimumab; sammenliknet med 9,1 mnd ved kjemoterapi alene. I hele studiepopulasjonen var OS hhv 13,2, 12,8 og 10,7 mnd. Tolv-måneders OS blant PD-L1 positive var 58 % for nivo/kjemo, 57 % for nivo/ipi og 37 % for kjemo. I hele studiepopulasjonen var tilsvarende 12-måneders overlevelse 54, 54 og 44 %. Det var også signifikant forlenget PFS i ITT populasjonen med hazard ratio 0,65 (p=0,0023).
Immunterapi i 1. linje ved HER-2 pos. AK i magesekk/GEJ
I den pågående Keynote-811 (placebokontrollert fase III studie) testes kjemoterapi i kombinasjon med trastuzumab +/- pembrolizumab (Chung et al., 2021). En interimanalyse presentert på ASCO 2021 viste lovende resultat med signifikant høyere objektiv responsrate med pembro på 74 vs. 52 % samt høyere andel komplette responser (11 vs. 3 %) (Yelena Y. Janjigian et al., 2021), noe som medførte at denne behandlingen ble godkjent av FDA. Endelige resultat og publikasjon avventes.
Andrelinje eller senere behandling
Sist faglig oppdatert: 28.06.2022
Cytostatikabehandling i 2. og senere linjer dersom immunterapi ikke skal benyttes
Som for behandling i 1.-linje, er de fleste studier av behandling i senere linjer dominert av pasienter med AK i GEJ og magesekk. Man har sett respons på cellegiftbehandling hos pasienter med PEK, men dokumentasjonen for gevinst av behandling i sistnevnte gruppe er ikke like god som for pasienter med AK.
Adenokarsinom (AK)
Irinotecan har vist økt overlevelse ved 2.-linjebehandling sammenlignet med «Best supportive care» (Thuss-Patience et al., 2011). Docetaxel viste i COUGAR-02-studien en OS på 5,2 mnd mot 3,6 mnd med «Best supportive care» (Ford et al., 2014). En fase III-studie viste ingen signifikant forskjell for OS mellom paclitaxel og irinotecan for pasienter tidligere behandlet med 5-FU og platinum-basert cellegift (Hironaka et al., 2013).
Ramucirumab er et monoklonalt antistoff som hemmer angiogenese via VGFR-2. Det foreligger to randomiserte studier hvor ramucirumab viser effekt på OS i 2.-linjebehandling. I REGARD-studien viste ramucirumab i monoterapi en OS på 5,2 mnd mot 3,8 mnd for placebogruppen (Fuchs et al., 2014). RAINBOW-studien sammenlignet paclitaxel alene med paclitaxel og ramucirumab i 2.-linje. Her viste kombinasjonsarmen en OS på 9,6 mnd mot 7,4 mnd for paclitaxel alene (Wilke et al., 2014).
I flere studier blir det rapportert at noen av pasientene får tredjelinjebehandling.
I en randomisert fase III-studie av pasienter med kjemo-refraktær (behandlet med minst to tidligere linjer kjemoterapi) AK i magesekk eller gastroøsofagealovergang, forbedret trifluridin / tipiracil total overlevelse (OS) sammenlignet med placebo (OS 5.7 mot 3,6 mnd., hazard ratio (HR) 0,69, P = 0,00058). (Shitara, Doi, et al., 2018).
Trifluridin/tipiracil kan vurderes i tredje linje for en selektert pasientgruppe i god funksjonsklasse (ECOG 0-I).
Det er for øvrige cellegifter ikke dokumentert at tredjelinjebehandling har bedre effekt enn beste lindrende behandling i en vestlig populasjon.
Plateepitelkarsinom (PEK)
Det finnes ikke studier som viser at cellgiftbehandling ved PEK i spiserør i 2. linje gir overlevelsesgevinst sammenlignet med BSC. Irinotekan (evt i kombinasjon med 5FU), paklitaxel og docetaxel anses likevel som aktuelle regimer etter progresjon på 5FU- og platinumbaserte regimer. Det er vist responsrater på opptil 25 % i studier hvor disse stoffene har vært brukt, enten mot hverandre (Yamamoto et al., 2021) eller sammenlignet med immunterapi (Kato et al., 2019; Kojima et al., 2020). Hos pasienter i god allmentilstand (ECOG 0-1) vil det kunne være meningsfullt å tilby et irinotekanbasert regime eller taxaner som 2.-linje cellegiftbehandling.
Immunterapi ved 2. og senere linje
Adenokarsinom (AK)
I KEYNOTE-061 fase III-studien (n = 592) (Shitara, Özgüroğlu, et al., 2018), ble pembrolizumab sammenlignet med paclitaxel i andrelinje ved avansert AK i magesekk eller GEJ. Pembrolizumab ga ikke signifikant bedret overlevelse sammenlignet med paclitaxel med PD-L1 CPS 1 eller høyere. I subgruppeanalyse var effekten av Pembrolizumab større for pasienter med PD-L1 CPS≥10 (HR 0,64; 95 % CI 0,41–1,02, median OS 10,4 måneder vs. 8 måneder).
KEYNOTE-059 fase II-studien, kohort 1, (Shitara, Özgüroğlu, et al., 2018) undersøkte pembrolizumab monoterapi ved avansert AK i magesekk eller GEJ hos 259 pasienter i 3. eller senere behandlingslinje. Den objektive responsraten (ORR) og sykdomskontrollraten (DCR) var henholdsvis 12 % og 27 %, og median OS var 5,6 måneder. ORR hadde en tendens til å være høyere i PD-L1-positiv (CPS ≥ 1) og MSI-svulster.
I en koreansk fase II-studie (Kim et al., 2018), ble 61 pasienter med metastatisk magesekkreft behandlet med pembrolizumab i 2. eller 3. linje. Hos pasienter med MSI eller Epstein Barr virus (EBV) positive svulster, var det dramatisk respons (ORR var 85,7 % i MSI og 100 % i EBV positive). For 55 PD-L1 positive pasienter (CPS ≥ 1 %), var ORR signifikant høyere (50.0 % mot 0.0 % for PD-L1 negative), P <0,001).
I ATTRACTION-2 ( en asiatisk fase III studie) (Kang et al., 2017) ble 493 pasienter med metastatisk eller ikke resektabel AK i magesekk eller GEJ randomisert (2:1) til behandling med Nivolumab eller placebo i 3. eller senere behandlingslinje. OS var signifikant lenger i nivolumabgruppen med median overlevelse 5,26 mndr mot 4,14 mndr i placebogruppen (HR 0,63, 95 % CI 0,51-0,78, p <0,0001). 12 mndr overlevelse var 26,2 % ved nivolumab og 10,9 % ved placebo.
Plateepitelkarsinom (PEK) og adenokarsinom (AK)
KEYNOTE-181 (Kojima et al., 2020) var en global fase III-studie som inkluderte pasienter med lokalavansert eller metastatisk AK eller PEK (69,3 %) i spiserør (inkl. Siewert 1) etter progresjon på 1. linjebehandling. Pasientene ble randomisert til monoterapi pembrolizumab eller kjemoterapi (paclitaxel, docetaxel eller irinotecan). Primære endeounkter var OS hos pasienter med PD-L1 CPS ≥ 10, pasienter med PEK og alle pasienter. Ved PD-L1 CPS ≥ 10 var median OS signifikant bedre med pembrolizumab vs. kjemoterapi (9,3 vs. 6,7 mnd., HR 0,69, p 0,0074). Hos pasienter med PEK var median OS 8,2 vs. 7,1 mnd (HR 0,78, p=0,0095). Det var ingen forskjell i median OS for hele pasientgruppen (7,1 vs. 7,1 mnd.). Det var signifikant mer toksisitet hos pasienter som mottok kjemoterapi.
Plateepitelkarsinom (PEK)
ATTRACTION-3 (Kato et al., 2019), en fase-3, global multisenterstudie, randomiserte 419 pasienter med avansert spiserørs PEK, og som hadde behandlingssvikt på, eller var intolerante for, tidligere behandling med platinum/fluoropyrimidin, til enten nivolumab eller taksanbasert kjemoterapi. Median OS ble siginifikat forbedret i nivolumab-armen sammenliget med kjemoterapi (10,9 mnd, vs. 8,4 mnd; HR 0,77). 38 (18 %) av 209 pasienter i nivolumab-armen hadde behandlingsrelaterte bivirkninger av grad 3 eller 4 sammenlignet med 131 (63 %) av 208 pasienter i cellegiftgruppen. Denne behandlingen er godkjent av det europeiske legemiddelbyrået og har således markedsføringstillatelse i Norge, men er avslått i Beslutningsforum.
HER2-rettet behandling i senere linje? Trastuzumabderukstekan
DESTINY-Gastric01 studien var en fase II randomisert studie gjennomført i Japan og Sør-Korea der 187 pasienter med HER2-positiv AK i magesekk eller GEJ og som hadde progrediert på minst to tidligere behandlingslinjer, inklusive trastuzumab, ble randomisert 2:1 til trastuzumabderukstekan eller klinikers valg av kjemoterapi (89 % irinotekan og 11 % paklitaksel). Trastuzumabderukstekan gruppen hadde signifikant høyere responsrate (51 vs. 14 %), signifikant lengre median OS (12,5 vs. 8,4 mnd. HR for død 0.59, 95 % CI 0.39-0.88) og median PFS (5,6 vs. 3,5 mnd. HR 0.47, 95 % CI 0.31-0.71) (Shitara, Bang, et al., 2020). Trastuzumabderukstekan er et humanisert anti-HER2 IgG1 monoklonalt antistoff kovalent bundet til derukstekan (topoisomerase I-hemmer). Viktigste bivirkninger registrert var myelosuppresjon og interstitiell lungesykdom. Behandlingen er foreløpig ikke godkjent i det europeiske legemiddelverket.
Anbefalinger
Pasienter i god almenntilstand og WHO 0–2 bør vurderes for cytostatikabehandling ev. i kombinasjon med immunterapi (evidensgrad A).
Førstelinjebehandling
Det finnes ikke ett kjemoterapi-regime som er å foretrekke framfor de andre.
Som hovedregel bør et tostoffs regime med et fluoropyrimidin i kombinasjon med oxaliplatin, irinotecan (ved adenokarsinom) eller et taxan vurderes.
Aktuelle regimer er Karboplatin/5FU, oksaliplatin/5FU, CapOx, FOLFOX, CiFU eller FLOX. FOLFIRI kan også være aktuelt ved adenokarsinom.
Ved HER2-positiv adenokarsinom (IHC 2+ og ISH+, eller IHC 3+) anbefales trastuzumab i kombinasjon med platinum og kapecitabin/5FU.
Ved PEK med TPS ≥ 1 % anbefales nivolumab + kjemoterapi, alternativt nivolumab i kombinasjon med ipiliumab.
Ved PEK med CPS ≥ 10 anbefales pembrolizumab+ kjemoterapi, alternativt nivolumab + kjemoterapi, alternativt nivolumab i kombinasjon med ipiliumab
Ved AK og CPS ≥ 5 anbefales nivolumab + kjemoterapi
Ved AK og CPS ≥ 10 anbefales pembrolizumab + kjemoterapi eller nivolumab + kjemoterapi
Ved MSI-pos/dMMR karsinom anbefales Pembrolizumab eller nivolumab som monoterapi eller som kombinasjonbehandling med kjemoterapi.
Andrelinje og senere behandling
Taksaner (paclitaxel eller docetaxel) hvis ikke brukt før eller irinotecan basert (FOLFIRI, FLIRI eller irinotekan monoterapi)
Tredjelinje/fjerdelinje kjemoterapi med trifluridin / tipiracil for AK kan vurderes for pasienter med ECOG 0–1.
Pasienter som er ECOG 0-1(2) og PD-L1 CPS ≥ 10, MSI eller EBV positive og som enda ikke har fått behandling med PD-1 hemmer, kan immunterapi med PD-1 hemmer vurderes, etter progresjon på kjemoterapi
For pasienter over 75 år eller yngre pasienter med redusert almenntilstand anbefales FLV, capecitabin monoterapi, irinotecan monoterapi, taksaner, evt FLOX/FOLFOX i redusert dose-regimet (evidensgrad D).
Prosess og metode for utarbeiding av retningslinjene
Hva er nasjonale retningslinjer
Sist faglig oppdatert: 28.06.2022
Nasjonal helseplan (2007–2010) (Nasjonal helseplan (2007-2010), 2006) slår fast at Helsedirektoratet innenfor rettslige rammer har en normerende rolle for helsetjenesten på tvers av helseregioner og tjenestenivå. Helsedirektoratet er derved eneste instans som har myndighet til å utarbeide nasjonale retningslinjer for helsetjenesten. Nasjonal helseplan gir Helsedirektoratet en koordinerende rolle for å utvikle overordnede rammer for kreftomsorgen sammen med de regionale helseforetakene, kommunene og andre relevante instanser.
Nasjonale retningslinjer fra Helsedirektoratet er å betrakte som anbefalinger og råd, basert på aktuell faglig kunnskap fremskaffet på en systematisk, kunnskapsbasert måte. De nasjonale retningslinjene gir uttrykk for hva som anses som god praksis på utgivelsestidspunktet og er ment som et hjelpemiddel ved de avveininger tjenesteyterne må gjøre for å oppnå forsvarlighet og god kvalitet i tjenesten (Helse og omsorgstjenester: kreft, 2012).
Nasjonale retningslinjer er ikke rettslig bindende, men skal som faglig normerende langt på vei være styrende for de valg som skal tas. Ved å følge oppdaterte nasjonale retningslinjer vil fagpersonell bidra til å oppfylle kravet om faglig forsvarlighet i lovverket. Dersom en velger løsninger som i vesentlig grad avviker fra nasjonale faglige retningslinjer, bør en dokumentere dette og kunne begrunne sine valg.
Kunnskapsbasert prosess
Sist faglig oppdatert: 28.06.2022
Helsedirektoratet legger til grunn at alle nasjonale retningslinjer skal være utarbeidet etter en metode med vekt på forskningsbasert kunnskap, brukermedvirkning og tverrfaglighet. Dokumentasjonen skal være tydelig og tilgjengelig og retningslinjene skal være, anvendelige og oppdaterte.
Det er en omfattende prosess å lage gode retningslinjer som tilfredsstiller krav til prosess, metode og transparens, som er det nivå Helsedirektoratet og andre liknende organisasjoner har lagt til grunn for utforming av anbefalinger.
Helsedirektoratet ønsker i arbeidet med nasjonale retningslinjer for kreftbehandling å bygge på det arbeid faggruppene tilsluttet Onkologisk Forum i en årrekke har gjort med å lage behandlingsveiledere /handlingsprogram. For å dekke behovet for detaljerte anbefalinger ved stråleterapi er det etablert et samarbeid med KVIST-gruppen (Kvalitetssikring i stråleterapi) ved Statens strålevern.
I dette retningslinjearbeidet har arbeidsgruppen gjort følgende for å sikre en god håndtering av kunnskapsgrunnlaget:
- I en tidlig fase av arbeidet har faggruppen avklart hva retningslinjene skal omfatte når det gjelder diagnostikk, behandling og oppfølging av spiserørskreft.
- Faggruppen har i samarbeid med Helsedirektoratet vurdert hvilke områder det har vært behov for kunnskapsstøtte fra Kunnskapssenteret. I forbindelse med de aktuelle retningslinjene ble det ikke funnet behov for egne kunnskapsoppsummeringer.
- Det ble søkt etter retningslinjer, meta-analyser og systematiske oversikter ved søk i Medline (PubMed). I tillegg ble det søkt i Cochrane- og HTA-databasene etter systematiske oversikter som ikke er indeksført i Medline. Forfatterne har søkt på temaet i PubMed og for øvrig i medisinsk litteratur.
- Kunnskapssenteret bisto ved utarbeidelsen av første versjonen i 2007 arbeidsgruppen med gradering av kunnskapsgrunnlaget.
Gradering av kunnskapsgrunnlaget
Sist faglig oppdatert: 28.06.2022
Ved utarbeiding av nasjonale retningslinjer stilles det krav om at all relevant kunnskap på området hentes frem, beskrives og vurderes på en systematisk og åpen måte.
I disse retningslinjene har man benyttet følgende graderingsmodell for å vise hvilket vitenskapelig grunnlag kunnskapen er basert på.
| Studietype | Evidensnivå | Gradering av anbefalinger |
|---|---|---|
| Kunnskap som bygger på systematiske oversikter og metaanalyser av randomiserte kontrollerte studier. | Nivå 1a | A |
| Kunnskap som bygger på minst én randomisert kontrollert studie. | Nivå 1b | |
| Kunnskap som bygger på minst én godt utformet kontrollert studie uten randomisering. | Nivå 2a | B |
| Kunnskap som bygger på minst én annen godt utformet kvasi‑eksperimentell studie uten randomisering. | Nivå 2b | |
| Kunnskap som bygger på godt utformede ikke eksperimentelle beskrivende studier, som sammenlignende studier, korrelasjonsstudier og case-studier. | Nivå 3 | C |
| Kunnskap som bygger på rapporter eller oppfatninger fra eksperter, komiteer og/eller klinisk ekspertise hos respekterte autoriteter | Nivå 4 | D |
I disse retningslinjene er kun kunnskapsgrunnlaget og ikke anbefalingene gradert.
Bakgrunn og arbeidsprosess
Sist faglig oppdatert: 28.06.2022
Disse nasjonale retningslinjene for diagnostikk, behandling og oppfølging av spiserørskreft er en del av et nasjonalt handlingsprogram for spiserørskreft. Handlingsprogrammet vil etter hvert suppleres med veiledere for allmennpraktikere, sykepleiere og andre faggruppers arbeid med kreftpasienter. Målet er å dekke hele pasientforløpet for kreftpasienter.
Utvikling av nasjonale handlingsprogrammene for kreftbehandling er et viktig tiltak under Nasjonal strategi for kreftområdet (2006–2009). Nasjonale handlingsprogrammer for kreftbehandling skal bidra til at det offentlige tilbudet i kreftomsorgen blir av god kvalitet og likeverdig over hele landet.
Nasjonale faggrupper tilsluttet Onkologisk Forum har i en årrekke arbeidet med og utviklet behandlingsveiledere og handlingsprogram for ulike krefttyper. Helsedirektoratet ønsket i arbeidet med nasjonale handlingsprogrammer som ledd i Nasjonal strategi for kreftområdet å ta utgangspunkt i og bygge på dette arbeidet. Helsedirektoratet rettet derfor i november 2005 en henvendelse til Norsk Gastointestinal Cancer Gruppe (NGICG) og ba om forslag til representanter til en arbeidsgruppe som skulle settes sammen av fagekspertise og med representanter fra Nasjonalt kunnskapssenter for helsetjenesten og Helsedirektoratet. Det ble bedt om at alle nødvendige faggrupper skulle være representert og at gruppen skulle bestå av fagfolk fra alle helseregioner. KVIST-gruppen ved Statens strålevern har i en arbeidsgruppe med medlemmer fra NGICG og fagspesialister i stråleterapi utarbeidet mer detaljerte anbefalinger om stråleterapi ved spiserørskreft. Disse anbefalingene vil bli lenket til de generelle retningslinjene for behandling av spiserørskreft. KVIST-gruppen er også representert i prosjektets referansegruppe. De regionale helseforetakene har medvirket i arbeidet gjennom representasjon i prosjektetsstyrings- og referansegruppe, samt ved mulighet til å kommentere arbeidsgruppenes sammensetning. De regionale helseforetakene ble også bedt om at fagfolk innenfor rammen av sin arbeidstid ble fristilt til retningslinjearbeidet, jf. Bestillerdokumentet fra Helse- og omsorgsdepartementet. Helsedirektoratet har hatt prosjektledelse og sekretariatsfunksjon.
Disse nasjonale retningslinjene for spiserørskreft er utarbeidet på følgende måte:
Det nasjonale handlingsprogrammet med retningslinjene for spiserørskreft ble første gang publisert 20. desember 2007. Retningslinjene ble da skrevet av en arbeidsgruppe fra Norsk Gastrointestinal cancergruppe med fagfolk oppnevnt fra RHFene. Før ferdigstilling var den første versjonen av handlingsprogrammet på høring hos RHFene, Den norske legeforeningen, Kreftforeningen og dens pasientorganisasjoner, og Norsk gastrointestinal cancergruppe (NGICG).
NGICGs faggruppe for spiserørskreft, NGICG-ØV, har hatt ansvaret for revisjonene av Nasjonalt handlingsprogram for spiserørskreft. Første revisjon, dvs 2. utgave av handlingsprogrammet, ble publisert 28. juni 2012.
I 2015 startet faggruppen arbeidet med andre revisjon, dvs 3. utgave av handlingsprogrammet. Helsedirektoratet ferdigstilte i samarbeid med faggruppen handlingsprogrammet i november 2015 og det ble publisert 17. august 2015.
Ytterligere revisjon av handlingsprgorammet i NGICG-ØVs faggruppe medførte en 4. utgave av handlingsprogrammet ble publisert 25. november 2015.
Faggruppen leverte desember 2019 utkast til revidert handlingsprogram til Helsedirektoratet. Helsedirektoratet ferdigstilte i januar 2020 handlingsprogrammet i samarbeid med faggruppen den 5. utgave av handlingsprogrammet som ble publisert 7. februar 2020.
Den foreliggende 6. revisjonen av handlingsprogrammet ble publisert 28. juni 2022.
Habilitet
Sist faglig oppdatert: 28.06.2022
Alle gruppens medlemmer ble i forbindelse med arbeidet bedt om å oppgi potensielle interessekonflikter. Ingen interessekonflikter ble oppgitt. Helsedirektoratet har vurdert arbeidsgruppens medlemmer som habile i forhold til utarbeiding av utkast til nasjonale retningslinjer for diagnostikk, behandling, og oppfølging av spiserørskreft.
Ressursmessige konsekvenser
Sist faglig oppdatert: 28.06.2022
De forslag som fremlegges ved disse retningslinjene, vil ikke føre til vesentlig økte kostnader for spesialisthelsetjenesten.
Oppdatering av retningslinjene
Sist faglig oppdatert: 28.06.2022
Utviklingen av ny behandling på kreftområdet er rask. Det kan være behov for å endre retningslinjene fordi behandling er «utdatert» eller at det er behov en prosess med vurdering av ny, ofte kostbar kreftbehandling.
Nasjonale retningslinjer for spiserørskreft vil oppdateres etter følgende prosess:
- NGICG melder fra til Helsedirektoratet om behov for å endre retningslinjene
- Oppdateringen utføres av en redaksjonskomité med representanter fra fagmiljøet, Nasjonalt kunnskapssenter for helsetjenester og Helsedirektoratet
- De oppdaterte retningslinjene vil foreligge på www.helsedirektoratet.no
Innholdet i Nasjonale retningslinjer for spiserørskreft vil vurderes årlig og om nødvendig oppdateres.
Referanser
Sist faglig oppdatert: 28.06.2022
Abela, J. E., Going, J. J., Mackenzie, J. F., McKernan, M., O'Mahoney, S., & Stuart, R. C. (2008). Systematic four-quadrant biopsy detects Barrett's dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol., 103(4), 850-855.
Agilent Technologies. (2018). PD-L1 IHC 22C3 pharmDx: Interpretation Manual – NSCLC. Santa Clara: Agilent Technologies. Hentet fra https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf
Agilent Technologies. (2019). PD-L1 IHC 22C3 pharmDx Interpretation Manual – NSCLC: for in Vitro diagnostic use. Santa Clara: Agilent Technologies. Hentet fra https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf
Al-Batran, S.-E., Homann, N., Pauligk, C., Goetze, T. O., Meiler, J., Kasper, S., Kopp, H.-G., Mayer, F., Haag, G. M., Luley, K., Lindig, U., Schmiegel, W., Pohl, M., Stoehlmacher, J., Folprecht, G., Probst, S., Prasnikar, N., Fischbach, W., Mahlberg, R., Trojan, J., Koenigsmann, M., Martens, U. M., Thuss-Patience, P., Egger, M., Block, A., Heinemann, V., Illerhaus, G., Moehler, M., Schenk, M., Kullmann, F., Behringer, D. M., Heike, M., Pink, D., Teschendorf, C., Löhr, C., Bernhard, H., Schuch, G., Rethwisch, V., von Weikersthal, L. F., Hartmann, J. T., Kneba, M., Daum, S., Schulmann, K., Weniger, J., Belle, S., Gaiser, T., Oduncu, F. S., Güntner, M., Hozaeel, W., Reichart, A., Jäger, E., Kraus, T., Mönig, S., Bechstein, W. O., Schuler, M., Schmalenberg, H., Hofheinz, R. D., & Investigators, F. A. (2019). Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet, 393(10184), 1948-1957. https://doi.org/10.1016/S0140-6736(18)32557-1
Al-Batran, S.-E., Homann, N., Schmalenberg, H., Kopp, H.-G., Haag, G. M., Luley, K. B., Schmiegel, W. H., Folprecht, G., Probst, S., Prasnikar, N., Thuss-Patience, P. C., Fischbach, W., Trojan, J., Koenigsmann, M., Pauligk, C., Goetze, T. O., Jaeger, E., Meiler, J., Schuler, M. H., & Hofheinz, R. (2017). Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 35(15_suppl), 4004. https://doi.org/10.1200/JCO.2017.35.15_suppl.4004
Al-Batran, S. E., Hartmann, J. T., Hofheinz, R., Homann, N., Rethwisch, V., Probst, S., Stoehlmacher, J., Clemens, M. R., Mahlberg, R., Fritz, M., Seipelt, G., Sievert, M., Pauligk, C., Atmaca, A., & Jäger, E. (2008). Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 19(11), 1882-1887. https://doi.org/10.1093/annonc/mdn403
Allum, W. H., Blazeby, J. M., Griffin, S. M., Cunningham, D., Jankowski, J. A., Wong, R., Association of Upper Gastrointestinal Surgeons of Great, B., Ireland, t. B. S. o. G., & the British Association of Surgical, O. (2011). Guidelines for the management of oesophageal and gastric cancer. Gut, 60(11), 1449-1472.
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I., & Langley, R. E. (2009). Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 27(30), 5062-5067. https://doi.org/10.1200/jco.2009.22.2083
Ba-Ssalamah, A., Matzek, W., Baroud, S., Bastati, N., Zacherl, J., Schoppmann, S. F., Hejna, M., Wrba, F., Weber, M., Herold, C. J., & Gore, R. M. (2011). Accuracy of hydro-multidetector row CT in the local T staging of oesophageal cancer compared to postoperative histopathological results. Eur Radiol, 21(11), 2326-2335. https://doi.org/10.1007/s00330-011-2187-2
Bang, Y. J., Van Cutsem, E., Feyereislova, A., Chung, H. C., Shen, L., Sawaki, A., Lordick, F., Ohtsu, A., Omuro, Y., Satoh, T., Aprile, G., Kulikov, E., Hill, J., Lehle, M., Ruschoff, J., & Kang, Y. K. (2010). Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet, 376(9742), 687-697. https://doi.org/10.1016/s0140-6736(10)61121-x
Barbour, A. P., Rizk, N. P., Gonen, M., Tang, L., Bains, M. S., Rusch, V. W., Coit, D. G., & Brennan, M. F. (2007). Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg., 246(1), 1-8. https://doi.org/10.1097/01.sla.0000255563.65157.d2
Bedenne, L., Michel, P., Bouche, O., Milan, C., Mariette, C., Conroy, T., Pezet, D., Roullet, B., Seitz, J. F., Herr, J. P., Paillot, B., Arveux, P., Bonnetain, F., & Binquet, C. (2007). Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 25(10), 1160-1168. https://doi.org/10.1200/jco.2005.04.7118
Bergquist, H., Wenger, U., Johnsson, E., Nyman, J., Ejnell, H., Hammerlid, E., Lundell, L., & Ruth, M. (2005). Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Dis.Esophagus., 18(3), 131-139.
Biere, S. S., van Berge Henegouwen, M. I., Maas, K. W., Bonavina, L., Rosman, C., Garcia, J. R., Gisbertz, S. S., Klinkenbijl, J. H., Hollmann, M. W., de Lange, E. S., Bonjer, H. J., van der Peet, D. L., & Cuesta, M. A. (2012). Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet, 379(9829), 1887-1892. https://doi.org/10.1016/s0140-6736(12)60516-9
Bosman, F. T., Carneiro, F., Hruban, R. H., & Theise, N. D. (red.). (2010). WHO Classification of Tumours of the Digestive System (4. utg.). (WHO Classification of Tumours Volume 3). Lyon: IARC.
Bozzetti, F. (2010). Nutritional support in patients with oesophageal cancer. Support Care Cancer, 18(Suppl 2), 41-50.
Braga, M., Ljungqvist, O., Soeters, P., Fearon, K., Weimann, A., & Bozzetti, F. (2009). ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr., 28(4), 378-386.
Brierley, J., Gospodarwicz, M. K., & Wittekind, C. (2017). TNM classification of malignant tumours (8. utg.). Chichester: John Wiley & Sons.
Bruzzi, J. F., Munden, R. F., Truong, M. T., Marom, E. M., Sabloff, B. S., Gladish, G. W., Iyer, R. B., Pan, T. S., Macapinlac, H. A., & Erasmus, J. J. (2007). PET/CT of esophageal cancer: its role in clinical management. Radiographics : a review publication of the Radiological Society of North America, Inc, 27(6), 1635-1652. https://doi.org/10.1148/rg.276065742
Burgart, L. J., Chopp, W. V., & Jain, D. (2021). Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus (Version 4.2.0.0 utg.). College of American Pathologists (CAP).
Burmeister, B. H., Dickie, G., Smithers, B. M., Hodge, R., & Morton, K. (2000). Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Archives of otolaryngology--head & neck surgery, 126(2), 205-208.
Burmeister, B. H., Thomas, J. M., Burmeister, E. A., Walpole, E. T., Harvey, J. A., Thomson, D. B., Barbour, A. P., Gotley, D. C., & Smithers, B. M. (2011). Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. European journal of cancer (Oxford, England : 1990), 47(3), 354-360. https://doi.org/10.1016/j.ejca.2010.09.009
Cancer Genome Atlas Research, N., Analysis Working Group: Asan, U., Agency, B. C. C., Brigham, Women’s, H., Broad, I., Brown, U., Case Western Reserve, U., Dana-Farber Cancer, I., Duke, U., Greater Poland Cancer, C., Harvard Medical, S., Institute for Systems, B., Leuven, K. U., Mayo, C., Memorial Sloan Kettering Cancer, C., National Cancer, I., Nationwide Children’s, H., Stanford, U., University of, A., University of, M., University of North, C., University of, P., University of, R., University of Southern, C., University of Texas, M. D. A. C. C., University of, W., Van Andel Research, I., Vanderbilt, U., Washington, U., Genome Sequencing Center: Broad, I., Washington University in St, L., Genome Characterization Centers, B. C. C. A., Broad, I., Harvard Medical, S., Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, U., University of North, C., University of Southern California Epigenome, C., University of Texas, M. D. A. C. C., Van Andel Research, I., Genome Data Analysis Centers: Broad, I., Brown, U., Harvard Medical, S., Institute for Systems, B., Memorial Sloan Kettering Cancer, C., University of California Santa, C., University of Texas, M. D. A. C. C., Biospecimen Core Resource: International Genomics, C., Research Institute at Nationwide Children’s, H., Tissue Source Sites: Analytic Biologic, S., Asan Medical, C., Asterand, B., Barretos Cancer, H., BioreclamationIvt, Botkin Municipal, C., Chonnam National University Medical, S., Christiana Care Health, S., Cureline, Duke, U., Emory, U., Erasmus, U., Indiana University School of, M., Institute of Oncology of, M., International Genomics, C., Invidumed, Israelitisches Krankenhaus, H., Keimyung University School of, M., Memorial Sloan Kettering Cancer, C., National Cancer Center, G., Ontario Tumour, B., Peter MacCallum Cancer, C., Pusan National University Medical, S., Ribeirão Preto Medical, S., St. Joseph’s, H., Medical, C., St. Petersburg Academic, U., Tayside Tissue, B., University of, D., University of Kansas Medical, C., University of, M., University of North Carolina at Chapel, H., University of Pittsburgh School of, M., University of Texas, M. D. A. C. C., Disease Working Group: Duke, U., Memorial Sloan Kettering Cancer, C., National Cancer, I., University of Texas, M. D. A. C. C., Yonsei University College of, M., Data Coordination Center, C. I., & Project Team: National Institutes of, H. (2017). Integrated genomic characterization of oesophageal carcinoma. Nature, 541(7636), 169-175. https://doi.org/10.1038/nature20805
Cancer Registry of Norway. (2021). Cancer in Norway 2020: cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway. Hentet fra https://www.kreftregisteret.no/globalassets/cancer-in-norway/2020/cin-2020.pdf
Cats, A., Jansen, E. P. M., van Grieken, N. C. T., Sikorska, K., Lind, P., Nordsmark, M., Meershoek-Klein Kranenbarg, E., Boot, H., Trip, A. K., Swellengrebel, H. A. M., van Laarhoven, H. W. M., Putter, H., van Sandick, J. W., van Berge Henegouwen, M. I., Hartgrink, H. H., van Tinteren, H., van de Velde, C. J. H., & Verheij, M. (2018). Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. The Lancet. Oncology, 19(5), 616-628. https://doi.org/10.1016/s1470-2045(18)30132-3
Cense, H. A., van Eijck, C. H., & Tilanus, H. W. (2006). New insights in the lymphatic spread of oesophageal cancer and its implications for the extent of surgical resection. Best Pract Res Clin Gastroenterol., 20(5), 893-906. https://doi.org/10.1016/j.bpg.2006.03.010
Chandrasoma, P., Wickramasinghe, K., Ma, Y., & DeMeester, T. (2007). Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis.Esophagus., 20(1), 36-41.
Cheung, H. C., Siu, K. F., & Wong, J. (1987). Is pyloroplasty necessary in esophageal replacement by stomach? A prospective, randomized controlled trial. Surgery, 102(1), 19-24.
Choi, J., Kim, S. G., Kim, J. S., Jung, H. C., & Song, I. S. (2010). Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surgical endoscopy, 24(6), 1380-1386. https://doi.org/10.1007/s00464-009-0783-x
Chung, H. C., Bang, Y. J., C, S. F., Qin, S. K., Satoh, T., Shitara, K., Tabernero, J., Van Cutsem, E., Alsina, M., Cao, Z. A., Lu, J., Bhagia, P., Shih, C. S., & Janjigian, Y. Y. (2021). First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol, 17(5), 491-501. https://doi.org/10.2217/fon-2020-0737
Conroy, T., Galais, M.-P., Raoul, J.-L., Bouché, O., Gourgou-Bourgade, S., Douillard, J.-Y., Etienne, P.-L., Boige, V., Martel-Lafay, I., Michel, P., Llacer-Moscardo, C., François, E., Créhange, G., Abdelghani, M. B., Juzyna, B., Bedenne, L., Adenis, A., Fédération Francophone de Cancérologie, D., & Group, U.-G. (2014). Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. The Lancet. Oncology, 15(3), 305-314. https://doi.org/10.1016/S1470-2045(14)70028-2
Conroy, T., Yataghene, Y., Etienne, P. L., Michel, P., Senellart, H., Raoul, J. L., Mineur, L., Rives, M., Mirabel, X., Lamezec, B., Rio, E., Le, P. E., Peiffert, D., & Adenis, A. (2010). Phase II randomised trial of chemoradiotherapy with FOLFOX4 or cisplatin plus fluorouracil in oesophageal cancer. Br J Cancer, 103(9), 1349-1355.
Cook, M. B., Kamangar, F., Whiteman, D. C., Freedman, N. D., Gammon, M. D., Bernstein, L., Brown, L. M., Risch, H. A., Ye, W., Sharp, L., Pandeya, N., Webb, P. M., Wu, A. H., Ward, M. H., Giffen, C., Casson, A. G., Abnet, C. C., Murray, L. J., Corley, D. A., Nyren, O., Vaughan, T. L., & Chow, W. H. (2010). Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. Journal of the National Cancer Institute, 102(17), 1344-1353. https://doi.org/10.1093/jnci/djq289
Cooper, J. S., Guo, M. D., Herskovic, A., Macdonald, J. S., Martenson, J. A., Jr., al-Sarraf, M., Byhardt, R., Russell, A. H., Beitler, J. J., Spencer, S., Asbell, S. O., Graham, M. V., & Leichman, L. L. (1999). Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA, 281(17), 1623-1627.
Cunningham, D., Allum, W. H., Stenning, S. P., Thompson, J. N., Van de Velde, C. J., Nicolson, M., Scarffe, J. H., Lofts, F. J., Falk, S. J., Iveson, T. J., Smith, D. B., Langley, R. E., Verma, M., Weeden, S., Chua, Y. J., & Participants, M. T. (2006). Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 355(1), 11-20. https://doi.org/10.1056/NEJMoa055531
Cunningham, D., Starling, N., Rao, S., Iveson, T., Nicolson, M., Coxon, F., Middleton, G., Daniel, F., Oates, J., & Norman, A. R. (2008). Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med, 358(1), 36-46.
D'Journo, X. B., & Thomas, P. A. (2014). Current management of esophageal cancer. Journal of thoracic disease, 6 Suppl 2, S253-264. https://doi.org/10.3978/j.issn.2072-1439.2014.04.16
Davies, A. R., Deans, D. A., Penman, I., Plevris, J. N., Fletcher, J., Wall, L., Phillips, H., Gilmour, H., Patel, D., de, B. A., & Paterson-Brown, S. (2006). The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis.Esophagus., 19(6), 496-503.
de Jonge, P. J., van Blankenstein, M., Grady, W. M., & Kuipers, E. J. (2014). Barrett's oesophagus: epidemiology, cancer risk and implications for management. Gut, 63(1), 191-202. https://doi.org/10.1136/gutjnl-2013-305490
Dehdashti, F., & Siegel, B. A. (2006). PET and PET/CT Imaging in Esophageal and Gastric Cancers. I: P. E. Valk, D. Delbeke, D. L. Bailey, D. W. Townsend, & M. N. Maisey (red.), Positron Emission Tomography: Clinical Practice (s. 165-180). London: Springer London. (Reprinted from).
Den norske patologforening. (2018). Veileder i biopsibesvarelse av maligne svulster (3. utgave (versjon 5.0 – 2018) utg.). [s.l.]: Den norske patologforening. Hentet fra https://www.legeforeningen.no/contentassets/961983a735a24fc5815dff5b3fa1ce01/veileder-i-besvarelse-av-maligne-svulster-2018-versjon-5.pdf
di Pietro, M., Fitzgerald, R. C., & BSG Barrett's guidelines working group. (2018). Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett's oesophagus with low-grade dysplasia. Gut, 67(2), 392-393. https://doi.org/10.1136/gutjnl-2017-314135
Dolan, J. P., Kaur, T., Diggs, B. S., Luna, R. A., Sheppard, B. C., Schipper, P. H., Tieu, B. H., Bakis, G., Vaccaro, G. M., Holland, J. M., Gatter, K. M., Conroy, M. A., Thomas, C. A., Jr., & Hunter, J. G. (2016). Significant understaging is seen in clinically staged T2N0 esophageal cancer patients undergoing esophagectomy. Dis.Esophagus., 29(4), 320-325. https://doi.org/10.1111/dote.12334
Enzinger, P. C., Burtness, B. A., Niedzwiecki, D., Ye, X., Douglas, K., Ilson, D. H., Villaflor, V. M., Cohen, S. J., Mayer, R. J., Venook, A., Benson, A. B., 3rd, & Goldberg, R. M. (2016). CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal Junction Cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 34(23), 2736-2742. https://doi.org/10.1200/JCO.2015.65.5092
Faglige anbefalinger for strålebehandling ved øsofaguscancer. (2009). Østerås: Statens strålevern. https://dsa.no/publikasjoner/stralevernrapport-7-2017-faglige-anbefalinger-for-kurativ-stralebehandling-ved-smacellet-lungecanser/StralevernRapport_7-2017_Faglige%20anbefalinger%20for%20SCLC.pdf
Far, A. E., Aghakhani, A., Hamkar, R., Ramezani, A., Pishbigar, H. F., Mirmomen, S., Roshan, M. R., Vahidi, S., Shahnazi, V., & Deljoodokht, Z. (2007). Frequency of human papillomavirus infection in oesophageal squamous cell carcinoma in Iranian patients. Scandinavian journal of infectious diseases, 39(1), 58-62. https://doi.org/10.1080/00365540600740496
Ferlay, J., Ervik, M., Lam, F., Colombet, M., Mery, L., Piñeros, M., Znaor, A., Soerjomataram, I., & Bray, F. Global Cancer Observatory: Cancer Today.Lyon: International Agency for Research on Cancer. fra https://gco.iarc.fr/today
Fitzgerald, R. C., di Pietro, M., Ragunath, K., Ang, Y., Kang, J. Y., Watson, P., Trudgill, N., Patel, P., Kaye, P. V., Sanders, S., O'Donovan, M., Bird-Lieberman, E., Bhandari, P., Jankowski, J. A., Attwood, S., Parsons, S. L., Loft, D., Lagergren, J., Moayyedi, P., Lyratzopoulos, G., & de Caestecker, J. (2014). British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut, 63(1), 7-42. https://doi.org/10.1136/gutjnl-2013-305372
Ford, H. E., Marshall, A., Bridgewater, J. A., Janowitz, T., Coxon, F. Y., Wadsley, J., Mansoor, W., Fyfe, D., Madhusudan, S., Middleton, G. W., Swinson, D., Falk, S., Chau, I., Cunningham, D., Kareclas, P., Cook, N., Blazeby, J. M., & Dunn, J. A. (2014). Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. The Lancet. Oncology, 15(1), 78-86. https://doi.org/10.1016/s1470-2045(13)70549-7
Fuchs, C. S., Tomasek, J., Yong, C. J., Dumitru, F., Passalacqua, R., Goswami, C., Safran, H., dos Santos, L. V., Aprile, G., Ferry, D. R., Melichar, B., Tehfe, M., Topuzov, E., Zalcberg, J. R., Chau, I., Campbell, W., Sivanandan, C., Pikiel, J., Koshiji, M., Hsu, Y., Liepa, A. M., Gao, L., Schwartz, J. D., & Tabernero, J. (2014). Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet, 383(9911), 31-39. https://doi.org/10.1016/s0140-6736(13)61719-5
Fumagali, U. (1996). Resective surgery for cancer of the thoracic esophagus. Results of a Consensus Conference held at the VIth World Congress of the International Society for Diseases of the Esophagus. Diseases of the Esophagus, 9 (Suppl. 1), 30-38.
Gkika, E., Gauler, T., Eberhardt, W., Stahl, M., Stuschke, M., & Pottgen, C. (2014). Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis.Esophagus., 27(7), 678-684. https://doi.org/10.1111/dote.12146
Guidelines for the diagnosis and management of Barrett's columnar-lined oesophagus. (2005). London: British Society of Gastroenterology. Hentet fra
https://www.bsg.org.uk/clinical-resource/bsg-guidelines-on-the-diagnosis-and-management-of-barretts-oesophagus/
Guimbaud, R., Louvet, C., Ries, P., Ychou, M., Maillard, E., Andre, T., Gornet, J. M., Aparicio, T., Nguyen, S., Azzedine, A., Etienne, P. L., Boucher, E., Rebischung, C., Hammel, P., Rougier, P., Bedenne, L., & Bouche, O. (2014). Prospective, randomized, multicenter, phase III study of fluorouracil, leucovorin, and irinotecan versus epirubicin, cisplatin, and capecitabine in advanced gastric adenocarcinoma: a French intergroup (Federation Francophone de Cancerologie Digestive, Federation Nationale des Centres de Lutte Contre le Cancer, and Groupe Cooperateur Multidisciplinaire en Oncologie) study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(31), 3520-3526. https://doi.org/10.1200/jco.2013.54.1011
Hagens, E. R. C., van Berge Henegouwen, M. I., Cuesta, M. A., & Gisbertz, S. S. (2017). The extent of lymphadenectomy in esophageal resection for cancer should be standardized. Journal of thoracic disease, 9(Suppl 8), S713-s723. https://doi.org/10.21037/jtd.2017.07.42
Hagens, E. R. C., van Berge Henegouwen, M. I., & Gisbertz, S. S. (2020). Distribution of Lymph Node Metastases in Esophageal Carcinoma Patients Undergoing Upfront Surgery: A Systematic Review. Cancers (Basel), 12(6). https://doi.org/10.3390/cancers12061592
Hagens, E. R. C., van Berge Henegouwen, M. I., van Sandick, J. W., Cuesta, M. A., van der Peet, D. L., Heisterkamp, J., Nieuwenhuijzen, G. A. P., Rosman, C., Scheepers, J. J. G., Sosef, M. N., van Hillegersberg, R., Lagarde, S. M., Nilsson, M., Räsänen, J., Nafteux, P., Pattyn, P., Hölscher, A. H., Schröder, W., Schneider, P. M., Mariette, C., Castoro, C., Bonavina, L., Rosati, R., de Manzoni, G., Mattioli, S., Garcia, J. R., Pera, M., Griffin, M., Wilkerson, P., Chaudry, M. A., Sgromo, B., Tucker, O., Cheong, E., Moorthy, K., Walsh, T. N., Reynolds, J., Tachimori, Y., Inoue, H., Matsubara, H., Kosugi, S. I., Chen, H., Law, S. Y. K., Pramesh, C. S., Puntambekar, S. P., Murthy, S., Linden, P., Hofstetter, W. L., Kuppusamy, M. K., Shen, K. R., Darling, G. E., Sabino, F. D., Grimminger, P. P., Meijer, S. L., Bergman, J., Hulshof, M., van Laarhoven, H. W. M., Mearadji, B., Bennink, R. J., Annema, J. T., Dijkgraaf, M. G. W., & Gisbertz, S. S. (2019). Distribution of lymph node metastases in esophageal carcinoma [TIGER study]: study protocol of a multinational observational study. BMC Cancer, 19(1), 662. https://doi.org/10.1186/s12885-019-5761-7
Haisley, K. R., Abdelmoaty, W. F., & Dunst, C. M. (2021). Laparoscopic Transhiatal Esophagectomy for Invasive Esophageal Adenocarcinoma. J Gastrointest Surg, 25(1), 9-15. https://doi.org/10.1007/s11605-019-04506-4
Halm, E. A., Lee, C., & Chassin, M. R. (2002). Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med., 137(6), 511-520.
Harada, K., Hwang, H., Wang, X., Abdelhakeem, A., Iwatsuki, M., Blum Murphy, M. A., Maru, D. M., Weston, B., Lee, J. H., Rogers, J. E., Thomas, I., Shanbhag, N., Zhao, M., Bhutani, M. S., Nguyen, Q. N., Swisher, S. G., Ikoma, N., Badgwell, B. D., Hofstetter, W. L., & Ajani, J. A. (2021). Frequency and Implications of Paratracheal Lymph Node Metastases in Resectable Esophageal or Gastroesophageal Junction Adenocarcinoma. Ann Surg., 273(4), 751-757. https://doi.org/10.1097/sla.0000000000003383
Hauge, T., Amdal, C. D., Falk, R. S., Johannessen, H.-O., & Johnson, E. (2020). Long-term outcome in patients operated with hybrid esophagectomy for esophageal cancer – a cohort study. Acta Oncologica, 59(7), 859-865. https://doi.org/10.1080/0284186X.2020.1750694
Haverkamp, L., Seesing, M. F., Ruurda, J. P., Boone, J., & R, V. H. (2017). Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis.Esophagus., 30(1), 1-7. https://doi.org/10.1111/dote.12480
Helse og omsorgstjenester: kreft. (5 mars 2012). [nettdokument]. Oslo: Helsedirektoratet. Hentet Mai 2012.
Hironaka, S., Ueda, S., Yasui, H., Nishina, T., Tsuda, M., Tsumura, T., Sugimoto, N., Shimodaira, H., Tokunaga, S., Moriwaki, T., Esaki, T., Nagase, M., Fujitani, K., Yamaguchi, K., Ura, T., Hamamoto, Y., Morita, S., Okamoto, I., Boku, N., & Hyodo, I. (2013). Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(35), 4438-4444. https://doi.org/10.1200/JCO.2012.48.5805
Hoeppner, J., Lordick, F., Brunner, T., Glatz, T., Bronsert, P., Rothling, N., Schmoor, C., Lorenz, D., Ell, C., Hopt, U. T., & Siewert, J. R. (2016). ESOPEC: prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer, 16, 503. https://doi.org/10.1186/s12885-016-2564-y
Hofmann, M., Stoss, O., Shi, D., Buttner, R., van de Vijver, M., Kim, W., Ochiai, A., Ruschoff, J., & Henkel, T. (2008). Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology, 52(7), 797-805. https://doi.org/10.1111/j.1365-2559.2008.03028.x
Homs, M. Y., Steyerberg, E. W., Eijkenboom, W. M., Tilanus, H. W., Stalpers, L. J., Bartelsman, J. F., van Lanschot, J. J., Wijrdeman, H. K., Mulder, C. J., Reinders, J. G., Boot, H., Aleman, B. M., Kuipers, E. J., & Siersema, P. D. (2005). Palliatieve behandeling voor slokdarmkanker met passageklachten: gunstiger uitkomsten van eenmalige inwendige brachytherapie dan van plaatsing van een zelfexpanderende stent; multicentrisch, gerandomiseerd onderzoek [Palliative treatment of esophageal cancer with dysphagia: more favourable outcome from single-dose internal brachytherapy than from the placement of a self-expanding stent; a multicenter randomised study]. Ned Tijdschr Geneeskd., 149(50), 2800-2806 [På nederlandsk].
Homs, M. Y. V., Steyerberg, E. W., Eijkenboom, W. M. H., Tilanus, H. W., Stalpers, L. J. A., Bartelsman, J. F. W. M., van Lanschot, J. J. B., Wijrdeman, H. K., Mulder, C. J. J., Reinders, J. G., Boot, H., Aleman, B. M. P., Kuipers, E. J., & Siersema, P. D. (2004). Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet, 364(9444), 1497-1504. https://doi.org/10.1016/S0140-6736(04)17272-3
Horgan, S., Berger, R. A., Elli, E. F., & Espat, N. J. (2003). Robotic-assisted minimally invasive transhiatal esophagectomy. The American surgeon, 69(7), 624-626.
Hosch, S. B., Stoecklein, N. H., Pichlmeier, U., Rehders, A., Scheunemann, P., Niendorf, A., Knoefel, W. T., & Izbicki, J. R. (2001). Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 19(7), 1970-1975. https://doi.org/10.1200/jco.2001.19.7.1970
Huang, S. H., Lockwood, G., Brierley, J., Cummings, B., Kim, J., Wong, R., Bayley, A., & Ringash, J. (2008). Effect of concurrent high-dose cisplatin chemotherapy and conformal radiotherapy on cervical esophageal cancer survival. International journal of radiation oncology, biology, physics, 71(3), 735-740. https://doi.org/10.1016/j.ijrobp.2007.10.022
Huang, Y., Wang, H., Luo, G., Zhang, Y., Wang, L., & Li, K. (2017). A systematic review and network meta-analysis of neoadjuvant therapy combined with surgery for patients with resectable esophageal squamous cell carcinoma. International journal of surgery (London, England), 38, 41-47. https://doi.org/10.1016/j.ijsu.2016.12.035
Hulscher, J. B., & van Lanschot, J. J. (2005). Individualised surgical treatment of patients with an adenocarcinoma of the distal oesophagus or gastro-oesophageal junction. Dig Surg., 22(3), 130-134.
Hulscher, J. B., van Sandick, J. W., de Boer, A. G., Wijnhoven, B. P., Tijssen, J. G., Fockens, P., Stalmeier, P. F., Ten Kate, F. J., van, D. H., Obertop, H., Tilanus, H. W., & van Lanschot, J. J. (2002). Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med, 347(21), 1662-1669.
Hulshof, M., Geijsen, E. D., Rozema, T., Oppedijk, V., Buijsen, J., Neelis, K. J., Nuyttens, J., van der Sangen, M. J. C., Jeene, P. M., Reinders, J. G., van Berge Henegouwen, M. I., Thano, A., van Hooft, J. E., van Laarhoven, H. W. M., & van der Gaast, A. (2021). Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 39(25), 2816-2824. https://doi.org/10.1200/jco.20.03697
International Agency for Research on Cancer. (2019). Digestive system tumours (5th ed. utg. Vol. Vol. 1). Lyon: International Agency for Research on Cancer.
Islami, F., Boffetta, P., Ren, J. S., Pedoeim, L., Khatib, D., & Kamangar, F. (2009). High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer, 125(3), 491-524. https://doi.org/10.1002/ijc.24445
Islami, F., Fedirko, V., Tramacere, I., Bagnardi, V., Jenab, M., Scotti, L., Rota, M., Corrao, G., Garavello, W., Schuz, J., Straif, K., Negri, E., Boffetta, P., & La Vecchia, C. (2011). Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer, 129(10), 2473-2484. https://doi.org/10.1002/ijc.25885
Islami, F., & Kamangar, F. (2008). Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res, 1(5), 329-338.
Jamieson, G. G., Lamb, P. J., & Thompson, S. K. (2009). The role of lymphadenectomy in esophageal cancer. Ann Surg., 250(2), 206-209.
Janjigian, Y. Y., Kawazoe, A., Yanez, P. E., Luo, S., Lonardi, S., Kolesnik, O., Barajas, O., Bai, Y., Shen, L., Tang, Y., Wyrwicz, L., Shitara, K., Qin, S., Cutsem, E. V., Tabernero, J., Li, L., Shih, C.-S., Bhagia, P., Chung, H. C. C., & investigators, o. b. o. t. K.-. (2021). Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. Journal of Clinical Oncology, 39(15_suppl), 4013-4013. https://doi.org/10.1200/JCO.2021.39.15_suppl.4013
Janjigian, Y. Y., Shitara, K., Moehler, M., Garrido, M., Salman, P., Shen, L., Wyrwicz, L., Yamaguchi, K., Skoczylas, T., Campos Bragagnoli, A., Liu, T., Schenker, M., Yanez, P., Tehfe, M., Kowalyszyn, R., Karamouzis, M. V., Bruges, R., Zander, T., Pazo-Cid, R., Hitre, E., Feeney, K., Cleary, J. M., Poulart, V., Cullen, D., Lei, M., Xiao, H., Kondo, K., Li, M., & Ajani, J. A. (2021). First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet, 398(10294), 27-40. https://doi.org/10.1016/s0140-6736(21)00797-2
Japanese Classification of Esophageal Cancer, 11th Edition: part I. (2017). Esophagus, 14(1), 1-36. https://doi.org/10.1007/s10388-016-0551-7
Jezerskyte, E., Mertens, A. C., van Dieren, S., Eshuis, W. J., Sprangers, M. A. G., van Berge Henegouwen, M. I., & Gisbertz, S. S. (2020). Gastrectomy Versus Esophagectomy For Gastroesophageal Junction Tumors: Short- And Long-term Outcomes From the Dutch Upper GI Cancer Audit. Ann Surg. https://doi.org/10.1097/sla.0000000000004610
Kamarajah, S. K., Phillips, A. W., Griffiths, E. A., Ferri, L., Hofstetter, W. L., & Markar, S. R. (2021). Esophagectomy or Total Gastrectomy for Siewert 2 Gastroesophageal Junction (GEJ) Adenocarcinoma? A Registry-Based Analysis. Ann Surg Oncol, 28(13), 8485-8494. https://doi.org/10.1245/s10434-021-10346-x
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., Chung, H. C., Chen, J. S., Muro, K., Kang, W. K., Yeh, K. H., Yoshikawa, T., Oh, S. C., Bai, L. Y., Tamura, T., Lee, K. W., Hamamoto, Y., Kim, J. G., Chin, K., Oh, D. Y., Minashi, K., Cho, J. Y., Tsuda, M., & Chen, L. T. (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 390(10111), 2461-2471. https://doi.org/10.1016/s0140-6736(17)31827-5
Kastelein, F., Spaander, M. C., Biermann, K., Steyerberg, E. W., Kuipers, E. J., & Bruno, M. J. (2011). Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett's esophagus. Gastroenterology, 141(6), 2000-2008; quiz e2013-2004. https://doi.org/10.1053/j.gastro.2011.08.036
Kato, K., Cho, B. C., Takahashi, M., Okada, M., Lin, C. Y., Chin, K., Kadowaki, S., Ahn, M. J., Hamamoto, Y., Doki, Y., Yen, C. C., Kubota, Y., Kim, S. B., Hsu, C. H., Holtved, E., Xynos, I., Kodani, M., & Kitagawa, Y. (2019). Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. The Lancet. Oncology, 20(11), 1506-1517. https://doi.org/10.1016/s1470-2045(19)30626-6
Kelly, R. J., Ajani, J. A., Kuzdzal, J., Zander, T., Van Cutsem, E., Piessen, G., Mendez, G., Feliciano, J., Motoyama, S., Lièvre, A., Uronis, H., Elimova, E., Grootscholten, C., Geboes, K., Zafar, S., Snow, S., Ko, A. H., Feeney, K., Schenker, M., Kocon, P., Zhang, J., Zhu, L., Lei, M., Singh, P., Kondo, K., Cleary, J. M., & Moehler, M. (2021). Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med, 384(13), 1191-1203. https://doi.org/10.1056/NEJMoa2032125
Keszei, A. P., Schouten, L. J., Goldbohm, R. A., & van den Brandt, P. A. (2012). Red and processed meat consumption and the risk of esophageal and gastric cancer subtypes in The Netherlands Cohort Study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 23(9), 2319-2326. https://doi.org/10.1093/annonc/mdr615
Khanna, L. G., & Gress, F. G. (2015). Preoperative evaluation of oesophageal adenocarcinoma. Best Pract Res Clin Gastroenterol., 29(1), 179-191. https://doi.org/10.1016/j.bpg.2014.12.005
Kidane, B., Coughlin, S., Vogt, K., & Malthaner, R. (2015). Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev., 5, CD001556. https://doi.org/10.1002/14651858.CD001556.pub3
Killeen, S. D., O'Sullivan, M. J., Coffey, J. C., Kirwan, W. O., & Redmond, H. P. (2005). Provider volume and outcomes for oncological procedures. Br J Surg., 92(4), 389-402.
Kim, S. T., Cristescu, R., Bass, A. J., Kim, K. M., Odegaard, J. I., Kim, K., Liu, X. Q., Sher, X., Jung, H., Lee, M., Lee, S., Park, S. H., Park, J. O., Park, Y. S., Lim, H. Y., Lee, H., Choi, M., Talasaz, A., Kang, P. S., Cheng, J., Loboda, A., Lee, J., & Kang, W. K. (2018). Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med, 24(9), 1449-1458. https://doi.org/10.1038/s41591-018-0101-z
Klevebro, F., Alexandersson von Dobeln, G., Wang, N., Johnsen, G., Jacobsen, A. B., Friesland, S., Hatlevoll, I., Glenjen, N. I., Lind, P., Tsai, J. A., Lundell, L., & Nilsson, M. (2016). A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 27(4), 660-667. https://doi.org/10.1093/annonc/mdw010
Kojima, T., Shah, M. A., Muro, K., Francois, E., Adenis, A., Hsu, C. H., Doi, T., Moriwaki, T., Kim, S. B., Lee, S. H., Bennouna, J., Kato, K., Shen, L., Enzinger, P., Qin, S. K., Ferreira, P., Chen, J., Girotto, G., de la Fouchardiere, C., Senellart, H., Al-Rajabi, R., Lordick, F., Wang, R., Suryawanshi, S., Bhagia, P., Kang, S. P., & Metges, J. P. (2020). Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 38(35), 4138-4148. https://doi.org/10.1200/jco.20.01888
Komeda, Y., Bruno, M., & Koch, A. (2014). EMR is not inferior to ESD for early Barrett's and EGJ neoplasia: An extensive review on outcome, recurrence and complication rates. Endoscopy international open, 2(2), E58-64. https://doi.org/10.1055/s-0034-1365528
Koyanagi, K., Kato, F., Kanamori, J., Daiko, H., Ozawa, S., & Tachimori, Y. (2018). Clinical significance of esophageal invasion length for the prediction of mediastinal lymph node metastasis in Siewert type II adenocarcinoma: A retrospective single-institution study. Ann Gastroenterol Surg, 2(3), 187-196. https://doi.org/10.1002/ags3.12069
Kranzfelder, M., Schuster, T., Geinitz, H., Friess, H., & Buchler, P. (2011). Meta-analysis of neoadjuvant treatment modalities and definitive non-surgical therapy for oesophageal squamous cell cancer. Br J Surg., 98(6), 768-783. https://doi.org/10.1002/bjs.7455
Kurokawa, Y., Hiki, N., Yoshikawa, T., Kishi, K., Ito, Y., Ohi, M., Wada, N., Takiguchi, S., Mine, S., Hasegawa, S., Matsuda, T., & Takeuchi, H. (2015). Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery, 157(3), 551-555. https://doi.org/10.1016/j.surg.2014.08.099
Lagergren, J., Bergstrom, R., Adami, H. O., & Nyren, O. (2000). Association between medications that relax the lower esophageal sphincter and risk for esophageal adenocarcinoma. Ann Intern Med., 133(3), 165-175.
Lagergren, J., Bergstrom, R., Lindgren, A., & Nyren, O. (1999). Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med, 340(11), 825-831.
Lagergren, J., Bergstrom, R., Lindgren, A., & Nyren, O. (2000). The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer, 85(3), 340-346.
Lagergren, J., Bergstrom, R., & Nyren, O. (1999). Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med., 130(11), 883-890.
Lagergren, J., Ye, W., Lagergren, P., & Lu, Y. (2010). The risk of esophageal adenocarcinoma after antireflux surgery. Gastroenterology, 138(4), 1297-1301. https://doi.org/10.1053/j.gastro.2010.01.004
Lai, A., Lipka, S., Kumar, A., Sethi, S., Bromberg, D., Li, N., Shen, H., Stefaniwsky, L., & Brady, P. (2018). Role of Esophageal Metal Stents Placement and Combination Therapy in Inoperable Esophageal Carcinoma: A Systematic Review and Meta-analysis. Digestive diseases and sciences, 63(4), 1025-1034. https://doi.org/10.1007/s10620-018-4957-z
Lee, Y. C., Marron, M., Benhamou, S., Bouchardy, C., Ahrens, W., Pohlabeln, H., Lagiou, P., Trichopoulos, D., Agudo, A., Castellsague, X., Bencko, V., Holcatova, I., Kjaerheim, K., Merletti, F., Richiardi, L., Macfarlane, G. J., Macfarlane, T. V., Talamini, R., Barzan, L., Canova, C., Simonato, L., Conway, D. I., McKinney, P. A., Lowry, R. J., Sneddon, L., Znaor, A., Healy, C. M., McCartan, B. E., Brennan, P., & Hashibe, M. (2009). Active and involuntary tobacco smoking and upper aerodigestive tract cancer risks in a multicenter case-control study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 18(12), 3353-3361. https://doi.org/10.1158/1055-9965.epi-09-0910
Leers, J. M., Knepper, L., van der Veen, A., Schröder, W., Fuchs, H., Schiller, P., Hellmich, M., Zettelmeyer, U., Brosens, L. A. A., Quaas, A., Ruurda, J. P., van Hillegersberg, R., & Bruns, C. J. (2020). The CARDIA-trial protocol: a multinational, prospective, randomized, clinical trial comparing transthoracic esophagectomy with transhiatal extended gastrectomy in adenocarcinoma of the gastroesophageal junction (GEJ) type II. BMC Cancer, 20(1), 781. https://doi.org/10.1186/s12885-020-07152-1
Leong, T., Smithers, B. M., Michael, M., Gebski, V., Boussioutas, A., Miller, D., Simes, J., Zalcberg, J., Haustermans, K., Lordick, F., Schuhmacher, C., Swallow, C., Darling, G., & Wong, R. (2015). TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer, 15, 532. https://doi.org/10.1186/s12885-015-1529-x
Levard, H., Pouliquen, X., Hay, J. M., Fingerhut, A., Langlois-Zantain, O., Huguier, M., Lozach, P., & Testart, J. (1998). 5-Fluorouracil and cisplatin as palliative treatment of advanced oesophageal squamous cell carcinoma. A multicentre randomised controlled trial. The French Associations for Surgical Research. Eur J Surg., 164(11), 849-857. https://doi.org/10.1080/110241598750005273
Li, X., Gao, C., Yang, Y., Zhou, F., Li, M., Jin, Q., & Gao, L. (2014). Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Alimentary pharmacology & therapeutics, 39(3), 270-281. https://doi.org/10.1111/apt.12574
Liu, J., Wang, J., Leng, Y., & Lv, C. (2013). Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer, 133(2), 473-485. https://doi.org/10.1002/ijc.28024
Lu, Z., Fang, Y., Liu, C., Zhang, X., Xin, X., He, Y., Cao, Y., Jiao, X., Sun, T., Pang, Y., Wang, Y., Zhou, J., Qi, C., Gong, J., Wang, X., Li, J., Tang, L., & Shen, L. (2021). Early Interdisciplinary Supportive Care in Patients With Previously Untreated Metastatic Esophagogastric Cancer: A Phase III Randomized Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 39(7), 748-756. https://doi.org/10.1200/jco.20.01254
Löfdahl, H. E., Lu, Y., Lagergren, P., & Lagergren, J. (2013). Risk factors for esophageal adenocarcinoma after antireflux surgery. Ann Surg., 257(4), 579-582. https://doi.org/10.1097/SLA.0b013e3182888384
Malik, S., Sharma, G., Sanaka, M. R., & Thota, P. N. (2018). Role of endoscopic therapy in early esophageal cancer. World journal of gastroenterology, 24(35), 3965-3973. https://doi.org/10.3748/wjg.v24.i35.3965
Malthaner, R. A., Collin, S., & Fenlon, D. (2006). Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst Rev., (3), CD001556.
Mannath, J., Subramanian, V., Hawkey, C. J., & Ragunath, K. (2010). Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett's esophagus: a meta-analysis. Endoscopy, 42(5), 351-359.
Maret-Ouda, J., Konings, P., Lagergren, J., & Brusselaers, N. (2016). Antireflux Surgery and Risk of Esophageal Adenocarcinoma: A Systematic Review and Meta-analysis. Ann Surg., 263(2), 251-257. https://doi.org/10.1097/sla.0000000000001438
Mariette, C., Dahan, L., Mornex, F., Maillard, E., Thomas, P. A., Meunier, B., Boige, V., Pezet, D., Robb, W. B., Le Brun-Ly, V., Bosset, J. F., Mabrut, J. Y., Triboulet, J. P., Bedenne, L., & Seitz, J. F. (2014). Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 32(23), 2416-2422. https://doi.org/10.1200/jco.2013.53.6532
Mariette, C., Markar, S. R., Dabakuyo-Yonli, T. S., Meunier, B., Pezet, D., Collet, D., D'Journo, X. B., Brigand, C., Perniceni, T., Carrère, N., Mabrut, J. Y., Msika, S., Peschaud, F., Prudhomme, M., Bonnetain, F., & Piessen, G. (2019). Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med, 380(2), 152-162. https://doi.org/10.1056/NEJMoa1805101
Mariette, C., Meunier, B., Pezet, D., Dalban, C., Collet, D., Thomas, P.-A., Brigand, C., Perniceni, T., Carrere, N., Bonnetain, F., & Piessen, G. (2015). Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol., 33(3_suppl), 5. https://doi.org/10.1200/jco.2015.33.3_suppl.5
Markar, S. R., Gronnier, C., Pasquer, A., Duhamel, A., Beal, H., Théreaux, J., Gagnière, J., Lebreton, G., Brigand, C., Meunier, B., Collet, D., Mariette, C., & AFC, F. w. g. F. (2016). Role of neoadjuvant treatment in clinical T2N0M0 oesophageal cancer: results from a retrospective multi-center European study. European journal of cancer (Oxford, England : 1990), 56, 59-68. https://doi.org/10.1016/j.ejca.2015.11.024
Markar, S. R., Mikhail, S., Malietzis, G., Athanasiou, T., Mariette, C., Sasako, M., & Hanna, G. B. (2016). Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg., 263(6), 1092-1101. https://doi.org/10.1097/sla.0000000000001542
Matsuda, S., Takeuchi, H., Kawakubo, H., & Kitagawa, Y. (2017). Three-field lymph node dissection in esophageal cancer surgery. Journal of thoracic disease, 9(Suppl 8), S731-S740. https://doi.org/10.21037/jtd.2017.03.171
Michalinos, A., Antoniou, S. A., Ntourakis, D., Schizas, D., Ekmektzoglou, K., Angouridis, A., & Johnson, E. O. (2020). Gastric ischemic preconditioning may reduce the incidence and severity of anastomotic leakage after οesophagectomy: a systematic review and meta-analysis. Dis.Esophagus., 33(10). https://doi.org/10.1093/dote/doaa010
Mine, S., Sano, T., Hiki, N., Yamada, K., Kosuga, T., Nunobe, S., & Yamaguchi, T. (2013). Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg., 100(8), 1050-1054. https://doi.org/10.1002/bjs.9170
Mine, S., Watanabe, M., Kumagai, K., Okamura, A., Yuda, M., Hayami, M., Yamashita, K., Imamura, Y., & Ishizuka, N. (2019). Comparison of mediastinal lymph node metastases from adenocarcinoma of the esophagogastric junction versus lower esophageal squamous cell carcinoma with involvement of the esophagogastric junction. Dis.Esophagus., 32(11). https://doi.org/10.1093/dote/doz002
Minsky, B. D., Pajak, T. F., Ginsberg, R. J., Pisansky, T. M., Martenson, J., Komaki, R., Okawara, G., Rosenthal, S. A., & Kelsen, D. P. (2002). INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 20(5), 1167-1174.
Mota, F. C., Cecconello, I., Takeda, F. R., Tustumi, F., Sallum, R. A. A., & Bernardo, W. M. (2018). Neoadjuvant therapy or upfront surgery? A systematic review and meta-analysis of T2N0 esophageal cancer treatment options. International journal of surgery (London, England), 54(Pt A), 176-181. https://doi.org/10.1016/j.ijsu.2018.04.053
Münch, S., Pigorsch, S. U., Devečka, M., Dapper, H., Weichert, W., Friess, H., Braren, R., Combs, S. E., & Habermehl, D. (2018). Comparison of definite chemoradiation therapy with carboplatin/paclitaxel or cisplatin/5-fluoruracil in patients with squamous cell carcinoma of the esophagus. Radiat Oncol, 13(1), 139. https://doi.org/10.1186/s13014-018-1085-z
Nafteux, P., Depypere, L., Van Veer, H., Coosemans, W., & Lerut, T. (2017). Principles of esophageal cancer surgery, including surgical approaches and optimal node dissection (2- vs. 3-field). Ann Cardiothorac Surg, 6(2), 152-158. https://doi.org/10.21037/acs.2017.03.04
Nasjonal faglig retningslinje for forebygging og behandling av underernæring. (2009). (IS-1580). Oslo: Helsedirektoratet. Hentet fra http://www.helsedirektoratet.no/publikasjoner/nasjonal-faglig-retningslinje-for-forebygging-og-behandling-av-underernering/Sider/default.aspx
Nasjonal helseplan (2007-2010). (2006). (Særtrykk av St.prp. nr. 1 (2006–2007) kapittel 6). Oslo: Helse- og omsorgsdepartemenetet. Hentet fra http://www.regjeringen.no/upload/HOD/Sykehus/Nasjonal_helseplan_Sartrykk.pdf
Nasjonalt kvalitetsregister for kreft i spiserør og magesekk. (2017). Årsrapport 2016 med resultater og forbedringstiltak. Oslo: Kreftregisteret. Hentet fra https://www.kreftregisteret.no/globalassets/publikasjoner-og-rapporter/arsrapporter/publisert-2017/arsrapport-2016_spiseror-og-magesekkreft.pdf
Norway, C. R. o. (2015). Cancer in Norway 2013: cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway. Hentet fra https://www.kreftregisteret.no/globalassets/cancer-in-norway/2013/cin_2013.pdf
Nuytens, F., Dabakuyo-Yonli, T. S., Meunier, B., Gagniere, J., Collet, D., D'Journo, X. B., Brigand, C., Perniceni, T., Carrere, N., Mabrut, J. Y., Msika, S., Peschaud, F., Prudhomme, M., Markar, S. R., Piessen, G., Federation de Recherche en, C., & French Eso-Gastric Tumors Working, G. (2021). Five-Year Survival Outcomes of Hybrid Minimally Invasive Esophagectomy in Esophageal Cancer: Results of the MIRO Randomized Clinical Trial. JAMA Surgery, 156(4), 323-332. https://doi.org/10.1001/jamasurg.2020.7081
Oesophageal and gastric cancers: diagnosis and assessment. (2001). Improving outcomes in upper gastro-intestinal cancers: the manual (s. 37-40). London: Department of Health. (Reprinted from). Hentet fra http://www.wales.nhs.uk/sites3/documents/362/UpperGIGuidance.pdf
Omloo, J. M., Lagarde, S. M., Hulscher, J. B., Reitsma, J. B., Fockens, P., van Dekken, H., Ten Kate, F. J., Obertop, H., Tilanus, H. W., & van Lanschot, J. J. (2007). Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg., 246(6), 992-1000.
Ovrebo, K. K., Lie, S. A., Laerum, O. D., Svanes, K., & Viste, A. (2012). Long-term survival from adenocarcinoma of the esophagus after transthoracic and transhiatal esophagectomy. World journal of surgical oncology, 10, 130. https://doi.org/10.1186/1477-7819-10-130
Owens, R., Cox, C., Gomberg, S., Pan, S., Radhakrishna, G., Parikh, S., Goody, R., Hingorani, M., Prince, S., Bird, T., Dorey, N., Macgregor, U., Al-Chamali, H., Hurt, C., & Mukherjee, S. (2020). Outcome of Weekly Carboplatin-Paclitaxel-based Definitive Chemoradiation in Oesophageal Cancer in Patients Not Considered to be Suitable for Platinum-Fluoropyrimidine-based Treatment: A Multicentre, Retrospective Review. Clin Oncol (R Coll Radiol), 32(2), 121-130. https://doi.org/10.1016/j.clon.2019.09.058
Pandeya, N., Webb, P. M., Sadeghi, S., Green, A. C., & Whiteman, D. C. (2010). Gastro-oesophageal reflux symptoms and the risks of oesophageal cancer: are the effects modified by smoking, NSAIDs or acid suppressants? Gut, 59(1), 31-38. https://doi.org/10.1136/gut.2009.190827
Pasquali, S., Yim, G., Vohra, R. S., Mocellin, S., Nyanhongo, D., Marriott, P., Geh, J. I., & Griffiths, E. A. (2017). Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg., 265(3), 481-491. https://doi.org/10.1097/sla.0000000000001905
Peng, J., Cai, J., Niu, Z. X., & Chen, L. Q. (2015). Early enteral nutrition compared with parenteral nutrition for esophageal cancer patients after esophagectomy: a meta-analysis. Dis.Esophagus., Feb 27 [Epub ahead of print]. https://doi.org/10.1111/dote.12337
Petrick, J. L., Wyss, A. B., Butler, A. M., Cummings, C., Sun, X., Poole, C., Smith, J. S., & Olshan, A. F. (2014). Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer, 110(9), 2369-2377. https://doi.org/10.1038/bjc.2014.96
Phoa, K. N., van Vilsteren, F. G., Weusten, B. L., Bisschops, R., Schoon, E. J., Ragunath, K., Fullarton, G., Di Pietro, M., Ravi, N., Visser, M., Offerhaus, G. J., Seldenrijk, C. A., Meijer, S. L., ten Kate, F. J., Tijssen, J. G., & Bergman, J. J. (2014). Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA, 311(12), 1209-1217. https://doi.org/10.1001/jama.2014.2511
Powell, N., Boyde, A., Tristram, A., Hibbitts, S., & Fiander, A. (2009). The potential impact of human papillomavirus vaccination in contemporary cytologically screened populations may be underestimated: an observational retrospective analysis of invasive cervical cancers. Int J Cancer, 125(10), 2425-2427. https://doi.org/10.1002/ijc.24571
Qu, X. M., Biagi, J. J., Hopman, W. M., & Mahmud, A. (2017). Shifting practice in definitive chemoradiation for localized esophageal cancer. Curr Oncol, 24(5), e379-e387. https://doi.org/10.3747/co.24.3677
Ragunath, K., Krasner, N., Raman, V. S., Haqqani, M. T., & Cheung, W. Y. (2003). A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett's esophagus. Endoscopy, 35(12), 998-1003.
Reynolds, J. V., Preston, S. R., O'Neill, B., Lowery, M. A., Baeksgaard, L., Crosby, T., Cunningham, M., Cuffe, S., Griffiths, G. O., Roy, R., Falk, S., Hanna, G., Bartlett, F. R., Parker, I., Alvarez-Iglesias, A., Nilsson, M., Piessen, G., Risum, S., Ravi, N., & McDermott, R. S. (2021). Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). (NCT01726452). Journal of Clinical Oncology, 39(15_suppl), 4004-4004. https://doi.org/10.1200/JCO.2021.39.15_suppl.4004
Ringe, K. I., Meyer, S., Ringe, B. P., Winkler, M., Wacker, F., & Raatschen, H. J. (2015). Value of oral effervescent powder administration for multidetector CT evaluation of esophageal cancer. European journal of radiology, 84(2), 215-220. https://doi.org/10.1016/j.ejrad.2014.11.008
Rizk, N. P., Ishwaran, H., Rice, T. W., Chen, L. Q., Schipper, P. H., Kesler, K. A., Law, S., Lerut, T. E., Reed, C. E., Salo, J. A., Scott, W. J., Hofstetter, W. L., Watson, T. J., Allen, M. S., Rusch, V. W., & Blackstone, E. H. (2010). Optimum lymphadenectomy for esophageal cancer. Ann Surg., 251(1), 46-50. https://doi.org/10.1097/SLA.0b013e3181b2f6ee
Rokkas, T., Pistiolas, D., Sechopoulos, P., Robotis, I., & Margantinis, G. (2007). Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 5(12), 1413-1417, 1417.e1411-1412. https://doi.org/10.1016/j.cgh.2007.08.010
Ronellenfitsch, U., Schwarzbach, M., Hofheinz, R., Kienle, P., Kieser, M., Slanger, T. E., Burmeister, B., Kelsen, D., Niedzwiecki, D., Schuhmacher, C., Urba, S., van de Velde, C., Walsh, T. N., Ychou, M., & Jensen, K. (2013). Preoperative chemo(radio)therapy versus primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. European journal of cancer (Oxford, England : 1990), 49(15), 3149-3158. https://doi.org/10.1016/j.ejca.2013.05.029
Ronellenfitsch, U., Schwarzbach, M., Hofheinz, R., Kienle, P., Kieser, M., Slanger, T. E., & Jensen, K. (2013). Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev., 5, CD008107. https://doi.org/10.1002/14651858.CD008107.pub2
Ross, P., Nicolson, M., Cunningham, D., Valle, J., Seymour, M., Harper, P., Price, T., Anderson, H., Iveson, T., Hickish, T., Lofts, F., & Norman, A. (2002). Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 20(8), 1996-2004. https://doi.org/10.1200/JCO.2002.08.105
Rubenstein, J. H., & Taylor, J. B. (2010). Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Alimentary pharmacology & therapeutics, 32(10), 1222-1227. https://doi.org/10.1111/j.1365-2036.2010.04471.x
Samson, P., Puri, V., Robinson, C., Lockhart, C., Carpenter, D., Broderick, S., Kreisel, D., Krupnick, A. S., Patterson, G. A., Meyers, B., & Crabtree, T. (2016). Clinical T2N0 Esophageal Cancer: Identifying Pretreatment Characteristics Associated With Pathologic Upstaging and the Potential Role for Induction Therapy. The Annals of thoracic surgery, 101(6), 2102-2111. https://doi.org/10.1016/j.athoracsur.2016.01.033
Sgourakis, G., Gockel, I., Radtke, A., Dedemadi, G., Goumas, K., Mylona, S., Lang, H., Tsiamis, A., & Karaliotas, C. (2010). The use of self-expanding stents in esophageal and gastroesophageal junction cancer palliation: a meta-analysis and meta-regression analysis of outcomes. Digestive diseases and sciences, 55(11), 3018-3030. https://doi.org/10.1007/s10620-010-1250-1
Shah, M. A., Janjigian, Y. Y., Stoller, R., Shibata, S., Kemeny, M., Krishnamurthi, S., Su, Y. B., Ocean, A., Capanu, M., Mehrotra, B., Ritch, P., Henderson, C., & Kelsen, D. P. (2015). Randomized Multicenter Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth Factor Support in Patients With Metastatic Gastric Adenocarcinoma: A Study of the US Gastric Cancer Consortium. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33(33), 3874-3879. https://doi.org/10.1200/jco.2015.60.7465
Shapiro, J., van Lanschot, J. J. B., Hulshof, M., van Hagen, P., van Berge Henegouwen, M. I., Wijnhoven, B. P. L., van Laarhoven, H. W. M., Nieuwenhuijzen, G. A. P., Hospers, G. A. P., Bonenkamp, J. J., Cuesta, M. A., Blaisse, R. J. B., Busch, O. R. C., Ten Kate, F. J. W., Creemers, G. M., Punt, C. J. A., Plukker, J. T. M., Verheul, H. M. W., Bilgen, E. J. S., van Dekken, H., van der Sangen, M. J. C., Rozema, T., Biermann, K., Beukema, J. C., Piet, A. H. M., van Rij, C. M., Reinders, J. G., Tilanus, H. W., Steyerberg, E. W., & van der Gaast, A. (2015). Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. The Lancet. Oncology, 16(9), 1090-1098. https://doi.org/10.1016/s1470-2045(15)00040-6
Shiozaki, H., Yano, M., Tsujinaka, T., Inoue, M., Tamura, S., Doki, Y., Yasuda, T., Fujiwara, Y., & Monden, M. (2001). Lymph node metastasis along the recurrent nerve chain is an indication for cervical lymph node dissection in thoracic esophageal cancer. Dis.Esophagus., 14(3-4), 191-196. https://doi.org/10.1046/j.1442-2050.2001.00206.x
Shitara, K., Bang, Y. J., Iwasa, S., Sugimoto, N., Ryu, M. H., Sakai, D., Chung, H. C., Kawakami, H., Yabusaki, H., Lee, J., Saito, K., Kawaguchi, Y., Kamio, T., Kojima, A., Sugihara, M., & Yamaguchi, K. (2020). Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med, 382(25), 2419-2430. https://doi.org/10.1056/NEJMoa2004413
Shitara, K., Doi, T., Dvorkin, M., Mansoor, W., Arkenau, H. T., Prokharau, A., Alsina, M., Ghidini, M., Faustino, C., Gorbunova, V., Zhavrid, E., Nishikawa, K., Hosokawa, A., Yalçın, Ş., Fujitani, K., Beretta, G. D., Cutsem, E. V., Winkler, R. E., Makris, L., Ilson, D. H., & Tabernero, J. (2018). Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Oncology, 19(11), 1437-1448. https://doi.org/10.1016/s1470-2045(18)30739-3
Shitara, K., Van Cutsem, E., Bang, Y. J., Fuchs, C., Wyrwicz, L., Lee, K. W., Kudaba, I., Garrido, M., Chung, H. C., Lee, J., Castro, H. R., Mansoor, W., Braghiroli, M. I., Karaseva, N., Caglevic, C., Villanueva, L., Goekkurt, E., Satake, H., Enzinger, P., Alsina, M., Benson, A., Chao, J., Ko, A. H., Wainberg, Z. A., Kher, U., Shah, S., Kang, S. P., & Tabernero, J. (2020). Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncology, 6(10), 1571-1580. https://doi.org/10.1001/jamaoncol.2020.3370
Shitara, K., Özgüroğlu, M., Bang, Y. J., Di Bartolomeo, M., Mandalà, M., Ryu, M. H., Fornaro, L., Olesiński, T., Caglevic, C., Chung, H. C., Muro, K., Goekkurt, E., Mansoor, W., McDermott, R. S., Shacham-Shmueli, E., Chen, X., Mayo, C., Kang, S. P., Ohtsu, A., & Fuchs, C. S. (2018). Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet, 392(10142), 123-133. https://doi.org/10.1016/s0140-6736(18)31257-1
Sikkema, M., de Jonge, P. J., Steyerberg, E. W., & Kuipers, E. J. (2010). Risk of esophageal adenocarcinoma and mortality in patients with Barrett's esophagus: a systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 8(3), 235-244; quiz e232. https://doi.org/10.1016/j.cgh.2009.10.010
Singh, S., Singh, A. G., Singh, P. P., Murad, M. H., & Iyer, P. G. (2013). Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett's esophagus: a systematic review and meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 11(6), 620-629. https://doi.org/10.1016/j.cgh.2012.12.036
Sjoquist, K. M., Burmeister, B. H., Smithers, B. M., Zalcberg, J. R., Simes, R. J., Barbour, A., & Gebski, V. (2011). Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. The Lancet. Oncology, 12(7), 681-692. https://doi.org/10.1016/s1470-2045(11)70142-5
Speicher, P. J., Ganapathi, A. M., Englum, B. R., Hartwig, M. G., Onaitis, M. W., D'Amico, T. A., & Berry, M. F. (2014). Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 9(8), 1195-1201. https://doi.org/10.1097/JTO.0000000000000228
Stahl, M., Stuschke, M., Lehmann, N., Meyer, H. J., Walz, M. K., Seeber, S., Klump, B., Budach, W., Teichmann, R., Schmitt, M., Schmitt, G., Franke, C., & Wilke, H. (2005). Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 23(10), 2310-2317. https://doi.org/10.1200/jco.2005.00.034
Stahl, M., Walz, M. K., Stuschke, M., Lehmann, N., Meyer, H. J., Riera-Knorrenschild, J., Langer, P., Engenhart-Cabillic, R., Bitzer, M., Konigsrainer, A., Budach, W., & Wilke, H. (2009). Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 27(6), 851-856. https://doi.org/10.1200/jco.2008.17.0506
Steber, C., Hughes, R. T., McTyre, E. R., Soike, M., Farris, M., Levine, B. J., Pasche, B., Levine, E., & Blackstock, A. W. (2021). Cisplatin/5-Fluorouracil (5-FU) Versus Carboplatin/Paclitaxel Chemoradiotherapy as Definitive or Pre-Operative Treatment of Esophageal Cancer. Cureus, 13(1), e12574. https://doi.org/10.7759/cureus.12574
Stockeld, D., Tennvall, J., Wagenius, G., Albertsson, M., Backman, L., Brodin, O., Cwikiel, M., Granstrom, L., Gustafsson, G., Gustavsson, S., Hambraeus, G., Lewensohn, R., Sjostedt, S., Strander, H., Aberg, B., & Fagerberg, J. (2001). A Swedish study of chemoradiation in squamous cell carcinoma of the esophagus. Acta Oncol, 40(5), 566-573.
Sun, J. M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., Kojima, T., Metges, J. P., Li, Z., Kim, S. B., Cho, B. C., Mansoor, W., Li, S. H., Sunpaweravong, P., Maqueda, M. A., Goekkurt, E., Hara, H., Antunes, L., Fountzilas, C., Tsuji, A., Oliden, V. C., Liu, Q., Shah, S., Bhagia, P., & Kato, K. (2021). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet, 398(10302), 759-771. https://doi.org/10.1016/s0140-6736(21)01234-4
Sun, L., & Yu, S. (2011). Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma. Dis.Esophagus., 24(8), 544-549. https://doi.org/10.1111/j.1442-2050.2011.01198.x
Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. (2002). Lancet, 359(9319), 1727-1733.
Takeuchi, H., & Kitagawa, Y. (2019). Sentinel node navigation surgery in esophageal cancer. Ann Gastroenterol Surg, 3(1), 7-13. https://doi.org/10.1002/ags3.12206
Taurchini, M., & Cuttitta, A. (2017). Minimally invasive and robotic esophagectomy: state of the art. Journal of visualized surgery, 3, 125. https://doi.org/10.21037/jovs.2017.08.23
Ter Veer, E., Haj Mohammad, N., van Valkenhoef, G., Ngai, L. L., Mali, R. M. A., Anderegg, M. C., van Oijen, M. G. H., & van Laarhoven, H. W. M. (2016). The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. Journal of the National Cancer Institute, 108(10), 10.1093/jnci/djw1166. https://doi.org/10.1093/jnci/djw166
Terheggen, G., Horn, E. M., Vieth, M., Gabbert, H., Enderle, M., Neugebauer, A., Schumacher, B., & Neuhaus, H. (2017). A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut, 66(5), 783-793. https://doi.org/10.1136/gutjnl-2015-310126
Thuss-Patience, P. C., Kretzschmar, A., Bichev, D., Deist, T., Hinke, A., Breithaupt, K., Dogan, Y., Gebauer, B., Schumacher, G., & Reichardt, P. (2011). Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). European journal of cancer (Oxford, England : 1990), 47(15), 2306-2314. https://doi.org/10.1016/j.ejca.2011.06.002
TNM Classification of malignant tumours. (2017). (8 utg.). Oxford,UK: Wiley-Blackwell.
Townsend, C. M. (2012). Sabiston textbook of surgery: the biological basis of modern surgical practice (19. utg.). Philadelphia, Pa.: Elsevier Saunders.
Tramacere, I., La Vecchia, C., & Negri, E. (2011). Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology (Cambridge, Mass.), 22(3), 344-349. https://doi.org/10.1097/EDE.0b013e31821092cd
Tramacere, I., Pelucchi, C., Bagnardi, V., Rota, M., Scotti, L., Islami, F., Corrao, G., Boffetta, P., La Vecchia, C., & Negri, E. (2012). A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 23(2), 287-297. https://doi.org/10.1093/annonc/mdr136
Turati, F., Tramacere, I., La Vecchia, C., & Negri, E. (2013). A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 24(3), 609-617. https://doi.org/10.1093/annonc/mds244
Ulla, M., Gentile, E. M., Cavadas, D., Yeyati, E. L., Frank, L., Argerich, J. I., & Garcia Monaco, R. (2012). Esophageal cancer characterization with pneumo-64-MDCT. Abdominal imaging, 37(4), 501-511. https://doi.org/10.1007/s00261-011-9784-z
Van Cutsem, E., Boni, C., Tabernero, J., Massuti, B., Middleton, G., Dane, F., Reichardt, P., Pimentel, F. L., Cohn, A., Follana, P., Clemens, M., Zaniboni, A., Moiseyenko, V., Harrison, M., Richards, D. A., Prenen, H., Pernot, S., Ecstein-Fraisse, E., Hitier, S., & Rougier, P. (2015). Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 26(1), 149-156. https://doi.org/10.1093/annonc/mdu496
Van Cutsem, E., Moiseyenko, V. M., Tjulandin, S., Majlis, A., Constenla, M., Boni, C., Rodrigues, A., Fodor, M., Chao, Y., Voznyi, E., Risse, M. L., & Ajani, J. A. (2006). Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 24(31), 4991-4997. https://doi.org/10.1200/jco.2006.06.8429
van Hagen, P., Hulshof, M. C., van Lanschot, J. J., Steyerberg, E. W., van Berge Henegouwen, M. I., Wijnhoven, B. P., Richel, D. J., Nieuwenhuijzen, G. A., Hospers, G. A., Bonenkamp, J. J., Cuesta, M. A., Blaisse, R. J., Busch, O. R., ten Kate, F. J., Creemers, G. J., Punt, C. J., Plukker, J. T., Verheul, H. M., Spillenaar Bilgen, E. J., van Dekken, H., van der Sangen, M. J., Rozema, T., Biermann, K., Beukema, J. C., Piet, A. H., van Rij, C. M., Reinders, J. G., Tilanus, H. W., & van der Gaast, A. (2012). Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med, 366(22), 2074-2084. https://doi.org/10.1056/NEJMoa1112088
van Meerten, E., Muller, K., Tilanus, H. W., Siersema, P. D., Eijkenboom, W. M., van Dekken, H., Tran, T. C., & van der Gaast, A. (2006). Neoadjuvant concurrent chemoradiation with weekly paclitaxel and carboplatin for patients with oesophageal cancer: a phase II study. Br J Cancer, 94(10), 1389-1394.
van Rijswijk, A. S., Hagens, E. R. C., van der Peet, D. L., van Berge Henegouwen, M. I., & Gisbertz, S. S. (2019). Differences in Esophageal Cancer Surgery in Terms of Surgical Approach and Extent of Lymphadenectomy: Findings of an International Survey. Ann Surg Oncol, 26(7), 2063-2072. https://doi.org/10.1245/s10434-019-07316-9
van Rossum, P. S., van Lier, A. L., Lips, I. M., Meijer, G. J., Reerink, O., van Vulpen, M., Lam, M. G., van Hillegersberg, R., & Ruurda, J. P. (2015). Imaging of oesophageal cancer with FDG-PET/CT and MRI. Clinical radiology, 70(1), 81-95. https://doi.org/10.1016/j.crad.2014.07.017
van Ruler, M. A., Peters, F. P., Slingerland, M., Fiocco, M., Grootenboers, D. A., Vulink, A. J., Marijnen, C. A., & Neelis, K. J. (2017). Clinical outcomes of definitive chemoradiotherapy using carboplatin and paclitaxel in esophageal cancer. Dis.Esophagus., 30(4), 1-9. https://doi.org/10.1093/dote/dow033
van Ruler, M. A. P., Peters, F. P., Slingerland, M., Fiocco, M., Grootenboers, D. A. R. H., Vulink, A. J. E., Marijnen, C. A. M., & Neelis, K. J. (2017). Clinical outcomes of definitive chemoradiotherapy using carboplatin and paclitaxel in esophageal cancer. Dis.Esophagus., 30(4), 1-9. https://doi.org/10.1093/dote/dow033
van Workum, F., Klarenbeek, B. R., Baranov, N., Rovers, M. M., & Rosman, C. (2020). Totally minimally invasive esophagectomy versus hybrid minimally invasive esophagectomy: systematic review and meta-analysis. Dis.Esophagus., 33(8). https://doi.org/10.1093/dote/doaa021
Visser, E., Markar, S. R., Ruurda, J. P., Hanna, G. B., & van Hillegersberg, R. (2019). Prognostic Value of Lymph Node Yield on Overall Survival in Esophageal Cancer Patients: A Systematic Review and Meta-analysis. Ann Surg., 269(2), 261-268. https://doi.org/10.1097/sla.0000000000002824
von Döbeln, G. A., Klevebro, F., Jacobsen, A. B., Johannessen, H. O., Nielsen, N. H., Johnsen, G., Hatlevoll, I., Glenjen, N. I., Friesland, S., Lundell, L., Yu, J., & Nilsson, M. (2019). Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long-term results of a randomized clinical trial. Dis.Esophagus., 32(2), 10.1093/dote/doy1078. https://doi.org/10.1093/dote/doy078
Wagner, A. D., Syn, N. L., Moehler, M., Grothe, W., Yong, W. P., Tai, B.-C., Ho, J., & Unverzagt, S. (2017). Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev., 8(8), CD004064. https://doi.org/10.1002/14651858.CD004064.pub4
Walterbos, N. R., Fiocco, M., Neelis, K. J., van der Linden, Y. M., Langers, A. M. J., Slingerland, M., de Steur, W. O., Peters, F. P., & Lips, I. M. (2019). Effectiveness of several external beam radiotherapy schedules for palliation of esophageal cancer. Clinical and Translational Radiation Oncology, 17, 24-31. https://doi.org/10.1016/j.ctro.2019.04.017
Wang, Z., Mao, Y., Gao, S., Li, Y., Tan, L., Daiko, H., Liu, S., Chen, C., Koyanagi, K., & He, J. (2020). Lymph node dissection and recurrent laryngeal nerve protection in minimally invasive esophagectomy. Annals of the New York Academy of Sciences, 1481(1), 20-29. https://doi.org/10.1111/nyas.14427
Webb, A., Cunningham, D., Scarffe, J. H., Harper, P., Norman, A., Joffe, J. K., Hughes, M., Mansi, J., Findlay, M., Hill, A., Oates, J., Nicolson, M., Hickish, T., O'Brien, M., Iveson, T., Watson, M., Underhill, C., Wardley, A., & Meehan, M. (1997). Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 15(1), 261-267. https://doi.org/10.1200/JCO.1997.15.1.261
Weimann, A., Braga, M., Harsanyi, L., Laviano, A., Ljungqvist, O., Soeters, P., Jauch, K. W., Kemen, M., Hiesmayr, J. M., Horbach, T., Kuse, E. R., & Vestweber, K. H. (2006). ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr., 25(2), 224-244.
Welsh, J., Settle, S. H., Amini, A., Xiao, L., Suzuki, A., Hayashi, Y., Hofstetter, W., Komaki, R., Liao, Z., & Ajani, J. A. (2012). Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer, 118(10), 2632-2640. https://doi.org/10.1002/cncr.26586
Wenger, U., Luo, J., Lundell, L., & Lagergren, J. (2005). A nationwide study of the use of self-expanding stents in patients with esophageal cancer in Sweden. Endoscopy, 37(4), 329-334.
Weusten, B., Bisschops, R., Coron, E., Dinis-Ribeiro, M., Dumonceau, J. M., Esteban, J. M., Hassan, C., Pech, O., Repici, A., Bergman, J., & di Pietro, M. (2017). Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy, 49(2), 191-198. https://doi.org/10.1055/s-0042-122140
Wilke, H., Muro, K., Van Cutsem, E., Oh, S. C., Bodoky, G., Shimada, Y., Hironaka, S., Sugimoto, N., Lipatov, O., Kim, T. Y., Cunningham, D., Rougier, P., Komatsu, Y., Ajani, J., Emig, M., Carlesi, R., Ferry, D., Chandrawansa, K., Schwartz, J. D., & Ohtsu, A. (2014). Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. The Lancet. Oncology, 15(11), 1224-1235. https://doi.org/10.1016/s1470-2045(14)70420-6
Winiker, M., Mantziari, S., Figueiredo, S. G., Demartines, N., Allemann, P., & Schafer, M. (2018). Accuracy of preoperative staging for a priori resectable esophageal cancer. Dis.Esophagus., 31(1), 1-6. https://doi.org/10.1093/dote/dox113
World Cancer Research Fund/American Institute for Cancer Research. (2018a). Diet, Nutrition, Physical Activity and Cancer: a Global Perspective:a summary of the Third Expert Report (Continous Update Project Expert Report 2018). London: World Cancer Research Fund/American Institute for Cancer Research. Hentet fra https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf
World Cancer Research Fund/American Institute for Cancer Research. (2018b). Diet, nutrition, physical and oesophageal cancer (Continuous Update Project Expert Report 2018). London. Hentet fra https://www.wcrf.org/wp-content/uploads/2021/02/oesophageal-cancer-report.pdf
Wu, A. H., Wan, P., & Bernstein, L. (2001). A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States). Cancer causes & control : CCC, 12(8), 721-732.
Wullstein, C., Ro-Papanikolaou, H. Y., Klingebiel, C., Ersahin, K., & Carolus, R. (2015). Minimally Invasive Techniques and Hybrid Operations for Esophageal Cancer. Viszeralmedizin, 31(5), 331-336. https://doi.org/10.1159/000438661
Wyss, A., Hashibe, M., Chuang, S. C., Lee, Y. C., Zhang, Z. F., Yu, G. P., Winn, D. M., Wei, Q., Talamini, R., Szeszenia-Dabrowska, N., Sturgis, E. M., Smith, E., Shangina, O., Schwartz, S. M., Schantz, S., Rudnai, P., Purdue, M. P., Eluf-Neto, J., Muscat, J., Morgenstern, H., Michaluart, P., Jr., Menezes, A., Matos, E., Mates, I. N., Lissowska, J., Levi, F., Lazarus, P., La Vecchia, C., Koifman, S., Herrero, R., Hayes, R. B., Franceschi, S., Wunsch-Filho, V., Fernandez, L., Fabianova, E., Daudt, A. W., Dal Maso, L., Curado, M. P., Chen, C., Castellsague, X., de Carvalho, M. B., Cadoni, G., Boccia, S., Brennan, P., Boffetta, P., & Olshan, A. F. (2013). Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. American journal of epidemiology, 178(5), 679-690. https://doi.org/10.1093/aje/kwt029
Xu, Y., Yu, X., Chen, Q., & Mao, W. (2012). Neoadjuvant versus adjuvant treatment: which one is better for resectable esophageal squamous cell carcinoma? World journal of surgical oncology, 10, 173. https://doi.org/10.1186/1477-7819-10-173
Xu, Y. J., Zhu, W. G., Liao, Z. X., Kong, Y., Wang, W. W., Li, J. C., Huang, R., He, H., Yang, X. M., Liu, L. P., Sun, Z. W., He, H. J., Bao, Y., Zeng, M., Pu, J., Hu, W. Y., Ma, J., Jiang, H., Liu, Z. G., Zhuang, T. T., Tan, B. X., Du, X. H., Qiu, G. Q., Zhou, X., Ji, Y. L., Hu, X., Wang, J., Ma, H. L., Zheng, X., Huang, J., Liu, A. W., Liang, X. D., Tao, H., Zhou, J. Y., Liu, Y., & Chen, M. (2020). [A multicenter randomized prospective study of concurrent chemoradiation with 60 Gy versus 50 Gy for inoperable esophageal squamous cell carcinoma]. Zhonghua Yi Xue Za Zhi, 100(23), 1783-1788. https://doi.org/10.3760/cma.j.cn112137-20200303-00574
Yamamoto, S., Kawakami, H., Kii, T., Hara, H., Kawabata, R., Kawada, J., Takeno, A., Matsuyama, J., Ueda, S., Okita, Y., Endo, S., Kimura, Y., Yanagihara, K., Okuno, T., Kurokawa, Y., Shimokawa, T., & Satoh, T. (2021). Randomized phase II study of docetaxel versus paclitaxel in patients with esophageal squamous cell carcinoma refractory to fluoropyrimidine- and platinum-based chemotherapy: OGSG1201. European journal of cancer (Oxford, England : 1990), 154, 307-315. https://doi.org/10.1016/j.ejca.2021.06.035
Yerokun, B. A., Sun, Z., Yang, C. J., Gulack, B. C., Speicher, P. J., Adam, M. A., D'Amico, T. A., Onaitis, M. W., Harpole, D. H., Berry, M. F., & Hartwig, M. G. (2016). Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. The Annals of thoracic surgery, 102(2), 416-423. https://doi.org/10.1016/j.athoracsur.2016.02.078
Yuan, L. G., & Mao, Y. S. (2019). Thoracic recurrent laryngeal nerve lymph node metastasis guides the cervical lymph node dissection of patients with esophageal cancer. Zhonghua Zhong Liu Za Zhi, 41(1), 10-14. https://doi.org/10.3760/cma.j.issn.0253-3766.2019.01.003
Zenda, S., Kojima, T., Kato, K., Izumi, S., Ozawa, T., Kiyota, N., Katada, C., Tsushima, T., Ito, Y., Akimoto, T., Hasegawa, Y., Kanamaru, M., & Daiko, H. (2016). Multicenter Phase 2 Study of Cisplatin and 5-Fluorouracil With Concurrent Radiation Therapy as an Organ Preservation Approach in Patients With Squamous Cell Carcinoma of the Cervical Esophagus. International journal of radiation oncology, biology, physics, 96(5), 976-984. https://doi.org/10.1016/j.ijrobp.2016.08.045
Zhang, Y. (2013). Epidemiology of esophageal cancer. World journal of gastroenterology, 19(34), 5598-5606. https://doi.org/10.3748/wjg.v19.i34.5598
Zhang, Z., Liao, Z., Jin, J., Ajani, J., Chang, J. Y., Jeter, M., Guerrero, T., Stevens, C. W., Swisher, S., Ho, L., Yao, J., Allen, P., Cox, J. D., & Komaki, R. (2005). Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. International journal of radiation oncology, biology, physics, 61(3), 656-664. https://doi.org/10.1016/j.ijrobp.2004.06.022
Zheng, R., Rios-Diaz, A. J., Liem, S., Devin, C. L., Evans, N. R., 3rd, Rosato, E. L., Palazzo, F., & Berger, A. C. (2021). Is the placement of jejunostomy tubes in patients with esophageal cancer undergoing esophagectomy associated with increased inpatient healthcare utilization? An analysis of the National Readmissions Database. Am J Surg., 221(1), 141-148. https://doi.org/10.1016/j.amjsurg.2020.06.028
Zhou, H.-Y., Zheng, S.-P., Li, A.-L., Gao, Q.-L., Ou, Q.-Y., Chen, Y.-J., Wu, S.-T., Lin, D.-G., Liu, S.-B., Huang, L.-Y., Li, F.-S., Zhu, H.-Y., Qiao, G.-B., Lanuti, M., Yao, H.-R., & Yu, Y.-F. (2020). Clinical evidence for association of neoadjuvant chemotherapy or chemoradiotherapy with efficacy and safety in patients with resectable esophageal carcinoma (NewEC study). EClinicalMedicine, 24, 100422. https://doi.org/10.1016/j.eclinm.2020.100422








