Nasjonalt handlingsprogram med retningslinjer for diagnostikk behandling og oppfølging av testikkelkreft
Forord
Sist faglig oppdatert: 23.05.2025
Nasjonale handlingsprogram med faglige retningslinjer for kreft skal bidra til at det offentlige tilbudet i kreftomsorgen blir av god kvalitet og likeverdig over hele landet. Målgrupper for retningslinjene er leger og legespesialister innen medisin, kirurgi, onkologi, radiologi, patologi og fastleger. De vil også være av interesse for andre faggrupper som er involvert i behandling og oppfølging av kreftpasienter og deres pårørende.
Nasjonale faglige retningslinjer fra Helsedirektoratet er å betrakte som anbefalinger og råd, basert på oppdatert faglig kunnskap. De nasjonale faglige retningslinjene gir uttrykk for hva som anses som god praksis på utgivelsestidspunktet, og er ment som et hjelpemiddel ved de avveininger tjenesteyterne må gjøre for å oppnå forvarlighet og god kvalitet i tjenesten. Nasjonale faglige retningslinjer er ikke direkte rettslig bindende for mottagerne, men bør langt på vei være styrende for de valg som skal tas. Ved å følge oppdaterte nasjonale faglige retningslinjer, vil fagpersonell bidra til å oppfylle kravet om faglig forsvarlighet. Dersom en velger løsninger som i vesentlig grad avviker fra de nasjonale faglige retningslinjene, bør en dokumentere dette, og være forberedt på å begrunne sine valg. Sykehusenes eiere og ledelse bør tilrettelegge virksomheten slik at de nasjonale faglige retningslinjene kan følges.
Helsedirektoratet takker arbeidsgruppen for stor innsats i utarbeidelsen av handlingsprogrammet. Vi håper handlingsprogrammet vil være et nyttig arbeidsredskap ved behandling av pasienter med testikkelkreft. Innholdet i disse nasjonale retningslinjene for testikkelkreft vil vurderes årlig, og om nødvendig oppdateres.
Disse nasjonale faglige retningslinjene for diagnostikk, behandling og oppfølging av tetikkelkreft er publisert mai 2025.
Bjørn Guldvog
Helsedirektør
Innledning
Generell informasjon
Sist faglig oppdatert: 15.04.2021
Testikkelkreft står for 1–2 % av alle maligniteter, men til tross for at den er så sjelden, er det den hyppigste kreftformen hos yngre menn. Insidensen er 6,2/100 000 i Vest-Europa og høyest i Norden unntatt Finland, med en insidens på 11,7/100 000 i Norge og 8,8/100 000 i Sverige. Det ble diagnostisert ca. 650 nye tilfeller i 2018, med 350 i Sverige og 317 i Norge (Norway, 2019; Socialstyrelsen, 2019).
95 % av all testikkelkreft er germinalcellesvulster, hvorav 55–60 % er seminomer og 40–45 % er non-seminomer. Høyeste insidens for seminompasienter er ved 38 års alder, som er ti år eldre enn for pasienter med non-seminom. Til tross for stor innsats er etiologien fortsatt ukjent, men sannsynligvis knyttet til aberrasjoner i utviklingen av gonadene (f.eks. kryptorkisme, dårlig sædkvalitet), noe som indikerer et tidlig første steg i onkogenesen i fosterlivet.
Seminomer har en monomorf patologi med én celletype avledet fra ekstraembryonalt vev. Derfor er rene seminomer ikke kompatible med forhøyede nivåer av alfaføprotein (AFP). Dette i motsetning til de komplekse non-seminomatøse germinalcelletumorene (NSGCT), som kan bestå av en blanding av forskjellige celletyper som stammer fra totipotent embryonalt vev, og også kan omfatte seminom-elementer.
Seminomer er biologisk forskjellige fra NSGCT, noe som reflekteres i den høyere forekomsten av sykdom på klinisk stadium (CS) I ved diagnose, som er 85 % sammenlignet med 60 % for NSGCT. Seminomer klassifiseres aldri i den dårlige prognosegruppen (3), se Appendix, Prognosegrupper etter IGCCCGfor prognosegrupper.
Til tross for at mange pasienter har metastatisk sykdom ved diagnosetidspunktet, er prognosen for testikkelkreft utmerket. Den relative overlevelsen etter 5 år er 98 % i Sverige og 98,8 % i Norge.
Behandlingsprotokoller og studier er nå primært fokusert på å redusere risikoen for alvorlige seneffekter uten å svekke behandlingsutfallet, og å utvikle behandlingen for å øke overlevelsen for de med dårligst prognose der nye behandlingskonsepter evalueres.
Formålet med kreftbehandlings¬programmet SWENOTECA X
Sist faglig oppdatert: 15.04.2021
Generelle formål
- Etablere et komplett register som omfatter alle mannlige pasienter (≥ 16 år) med testikkelkreft, retroperitoneale og mediastinale germinalcellekreft i Norge og Sverige
- Standardisere diagnostiske prosedyrer, stadiebestemmelse, behandling og oppfølging for å:
- Forbedre pasientutfallet
- Sikre prospektiv populasjonsbasert klinisk forskning av høy kvalitet
Spesifikke fokusområder i klinisk stadium I
Seminom
- Verifisere rapportert lav tilbakefallsrate uten adjuvant kjemoterapi hos pasienter med testikkelsvulst ≤ 4 cm og ingen stromal invasjon av rete testis
- Evaluere tilbakefallsraten henholdsvis med og uten adjuvant kjemoterapi hos pasienter med testikkelsvulst ≤ 4 cm og/eller stromal invasjon av rete testis
- Redusere oppfølgingsplanen ytterligere avhengig av risiko og tilbakefallsmønster
- Evaluere tidlig og sen toksisitet etter én kur adjuvant karboplatin
Non-seminom
- Risikotilpasset behandling: adjuvant kjemoterapi med én kur BEP, eller overvåkning
- Tilbakefallsfrekvens og -mønster for pasienter med antatt henholdsvis lav og høy risiko
- Tidlig og sen toksisitet etter henholdsvis adjuvant kjemoterapi versus behandling ved tilbakefall
Spesifikke fokusområder ved metastatisk sykdom
Seminom
- Evaluere terapeutisk effekt og tidlig og sen toksisitet ved BEP kjemoterapi
- Evaluere effekten av primærkirurgi ved non-bulky seminom CS IIA + IIB ≤ 3 cm, med
1–2 metastatiske lymfeknuter - Evaluere sensitiviteten og spesifisiteten til FDG-PET i ovennevnte setting
- Evaluere de tidlige og langsiktige bivirkningene av primærkirurgi
Non-seminom
- Individualisert behandling av metastatisk sykdom i henhold til risikogruppe og initialt fall i tumormarkører
- Redusere overbehandling der det er mulig, og intensivere behandlingen hos pasienter i intermediær eller dårlig prognosegruppe eller hos pasienter som responderer dårlig
- Evaluere primærkirurgi hos pasienter med markørnegativt klinisk stadium II A
- Evaluere behandlingsutfall, tid til tilbakefall, den histologiske typen av tilbakefallet og responsen på «salvage»-behandling
- Evaluere tidlige og sene bivirkninger etter behandling for avansert sykdom
Forkortelser
Sist faglig oppdatert: 15.04.2021
|
AFP |
Alfaføtoprotein |
|
AUC |
Areal under kurven |
|
BED |
Biologisk effektiv dose |
|
BEP |
Bleomycin, etoposid, cisplatin |
|
BIP |
Bleomycinindusert pneumonitt |
|
CE |
Karboplatin, etoposid |
|
CR |
Fullstendig remisjon |
|
CS |
Klinisk stadium |
|
CSS |
Kreftspesifikk overlevelse |
|
CT |
Computertomografi |
|
eGFR |
Estimert glomerulær filtrasjonshastighet |
|
EAU |
European Association of Urology |
|
EGCT |
Ekstragonadal germinalcellekreft |
|
EGCCCG |
European Germ cell Cancer Consensus Group |
|
EMA-CO |
Etoposid, metotreksat, dactinomycin, cyklofosfamid, vinkristin |
|
EP |
Etoposid, cisplatin |
|
FDG-PET |
Positronemisjonstomografi med fluorodeoksyglukose |
|
FSH |
Follikkelstimulerende hormon |
|
FU |
Oppfølging |
|
GCNIS |
Germinalcelleneoplasi in situ |
|
GCT |
Germinalcellekreft |
|
G-CSF |
Granulocytt-kolonistimulerende faktor |
|
GFR |
Glomerulær filtrasjonshastighet |
|
GOP |
Gemcitabin, oxaliplatin, paklitaksel |
|
ß-hCG |
Humant choriogonadotropin type beta |
|
HDCT |
Høydose kjemoterapi |
|
IGCCCG |
International Germ Cell Cancer Collaborative Group |
|
IPFSC |
International Prognostic Factors Study Group |
|
LDH |
Laktatdehydrogenase |
|
LH |
Luteiniserende hormon |
|
LVI |
Lymfovaskulær invasjon |
|
MRC |
Medical Research Council |
|
MRI |
Magnetresonanstomografi |
|
NSGCT |
Non-seminomatøs germinalcelletumor |
|
OS |
Total overlevelse |
|
PC-RPLND |
Ekstirpasjon av retroperitonealt restvev etter kjemoterapi for metastatisk sykdom |
|
PEI |
Cisplatin, etoposid, ifosfamid |
|
PFI |
Progresjonsfritt intervall |
|
PFS |
Progresjonsfri overlevelse |
|
PLAP |
Placentaliknende alkalisk fosfatase |
|
RMH |
Royal Marsden Hospital |
|
RPLND |
Retroperitoneal lymfeknutedisseksjon |
|
SGCT |
Seminomatøs germinalcelletumor |
|
SHBG |
Kjønnshormonbindende globulin |
|
SIB |
Simultan integrert boost |
|
SRT |
Stereotaktisk strålebehandling |
|
SWENOTECA |
Svensk-norsk testikkelkreftgruppe |
|
TCS |
Testikkelkreft-overlevere |
|
TGCC |
Testikulær germinalcellekreft |
|
TM |
Tumormarkører |
|
TIP |
Paklitaksel, ifosfamid og cisplatin |
|
ULN |
Øvre normalgrense |
|
VMAT |
Volumetric Modulated Arc Therapy |
|
WBRT |
Helhjernestråling |
|
WHO |
Verdens helseorganisasjon |
Forløpstider
Om pakkeforløp for kreft
Sist faglig oppdatert: 15.04.2021
Pakkeforløp for kreft skal gi forutsigbarhet og trygghet for pasient og pårørende, og er et standard pasientforløp som beskriver organisering av utredning og behandling, kommunikasjon/dialog med pasient og pårørende samt ansvarsplassering og konkrete forløpstider. Pakkeforløpet starter når et helseforetak eller privat ideelt sykehus mottar en henvisning med begrunnet mistanke om kreft, eller når helseforetaket selv starter utredning med begrunnet mistanke om kreft.
Formålet med Pakkeforløp for kreft er at kreftpasienter skal oppleve et godt organisert, helhetlig og forutsigbart forløp uten unødvendig ikke-medisinsk begrunnet forsinkelse i utredning, diagnostikk, behandling og rehabilitering.
Forløpstidene i pakkeforløpet beskriver den maksimale tiden de ulike fasene i forløpet bør ta. Forløpstidene angis i kalenderdager. De enkelte fasenes forløpstid legges til slutt sammen til en samlet forløpstid, som angir tiden fra henvisning er mottatt til behandling er startet. Med utgangspunkt i pakkeforløpet skal et individuelt forløp tilrettelegges for hver enkelt pasient.
De regionale helseforetakene har det overordnede ansvaret for å sikre at pakkeforløpene med forløpstidene blir implementert og fulgt opp. Forløpstidene er normerende og er ikke en pasientrettighet. Fortsatt er det lovmessige grunnlaget pasientrettighetsloven (Lov om pasient- og brukerrettigheter (pasient- og brukerrettighetsloven). LOV-1999-07-02-63) § 2‑2 og forskrift om prioritering av helsetjenester (Forskrift om prioritering av helsetjenester, rett til nødvendig helsehjelp fra spesialisthelsetjenesten, rett til behandling i utlandet og om klagenemnd (prioriteringsforskriften). FOR-2000-12-01-1208). Av og til vil det av faglige grunner være noen pasienter som ikke kan utredes ferdig innen normert forløpstid for oppstart av første behandling. Årsaker til avvik fra de normerte forløpstidene bør dokumenters i pasientjournalen.
Forløpstider for testikkelkreft
Sist faglig oppdatert: 15.04.2021
| Forløpsbeskrivelse | Forløpstid | |
|---|---|---|
| Fra henvisning mottatt til første fremmøte utredende avdeling | 5 kalenderdager | |
| Fra første fremmøte i utredende avdeling til avsluttet utredning (beslutning tas) | 12 kalenderdager | |
| Fra avsluttet utredning til start behandling | Kirurgisk behandling | 14 kalenderdager |
| Fra avsluttet utredning til start behandling | Medikamentell behandling | 21* kalenderdager |
| Fra henvisning mottatt til start behandling | Strålebehandling | 21 kalenderdager |
| Fra avsluttet utredning til start behandling | Beslutning og aktiv overvåkning (surveillance) eller beslutning om ny staging | 14 kalenderdager |
| Fra henvisning mottatt til start behandling | Kirurgisk behandling | 31 kalenderdager |
| Fra henvisning mottatt til start behandling | Medikamentell behandling | 38* kalenderdager |
| Fra henvisning mottatt til start behandling | Kirurgisk behandling | 38 kalenderdager |
| Fra henvisning mottatt til start behandling | Beslutning og aktiv overvåkning (surveillance) eller beslutning om ny staging | 31 kalenderdager |
* Ved metastatisk sykdom bør medikamentell behandling starte innen 14 kalenderdager.
Pakkeforløp for testikkelkreft finnes på Helsedirektoratets nettsider.
Det er utarbeidet egne diagnoseveiledere for fastleger for inngang til pakkeforløp. Diagnoseveileder for testikkelkreft finnes på Helsedirektoratets nettsider.
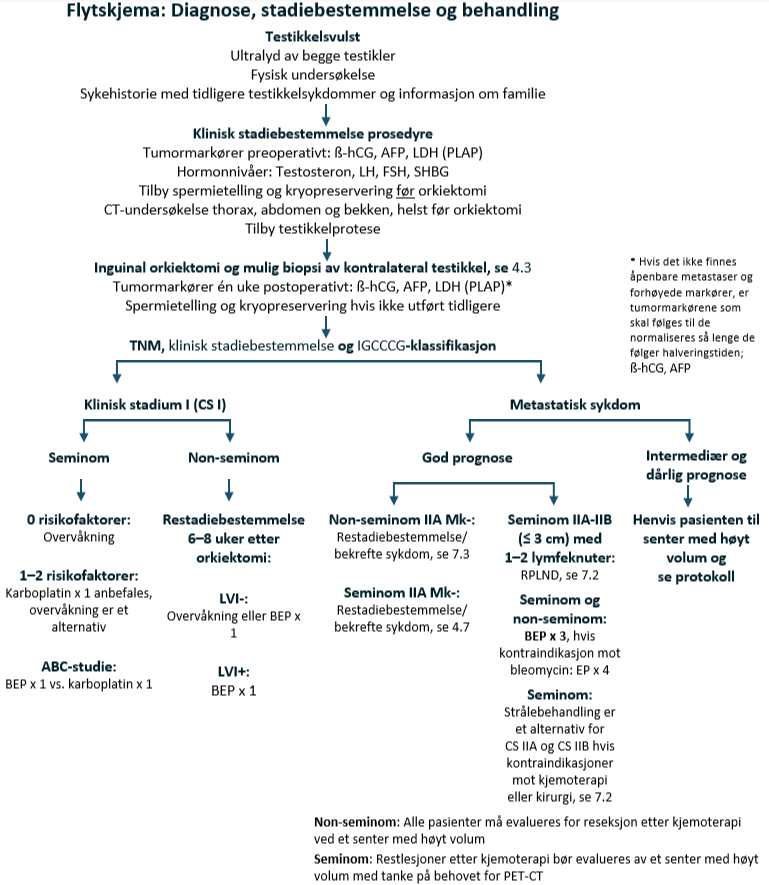
Diagnose og klinisk stadiebestemmelse
Diagnose
Sist faglig oppdatert: 23.05.2025
Prosedyrer før orkiektomi
- Ultralydundersøkelse av begge testikler med volummålinger i ml
- Generell fysisk undersøkelse
- Serumnivåer av AFP, ß-hCG, LDH
- Serumnivåer av PLAP, valgfritt
- Serumnivåer av LH, FSH, testosteron og SHBG
- CT-undersøkelse av thorax, abdomen og bekken
- Pasienter skal tilbys Spermieanalyse med kryopreservering før orkiektomi
- Pasienter skal tilbys testikkelprotese
- Historie med tidligere testikkelsykdom og informasjon om familie
Klinisk undersøkelse av testikkelen
Testikkelkreft presenterer seg vanligvis som en smertefri, ensidig kul i testikkelen og blir i de fleste tilfeller diagnostisert ved palpasjon. Omtrent 10 % av pasientene vil presentere seg med kliniske symptomer som ligner epididymitt. Det skal utføres ultralyd av begge testiklene, og eksplorasjon skal utføres ved alle tilfeller når kliniske undersøkelser eller ultralydundersøkelser ikke kan utelukke en svulst.
Tumormarkører i serum
Ved non-seminom har ca. 40 % av pasientene med CS I og opptil 85 % av metastatiske pasienter en økning i enten én eller begge tumormarkører i serum av AFP og ß-hCG (Ehrlich, Beck, Foster, Bihrle, & Einhorn, 2013). Seminom-pasienter mangler derimot ofte forhøyede tumormarkører. Markørkonsentrasjonen avhenger av histologisk subtype og tumorbyrde. Det forekommer et forhøyet nivå av LDH hos testikkelkreftpasienter, og det brukes også som en prognostisk markør.
AFP og ß-hCG måles og brukes for å:
- Identifisere okkult spredning (radiologisk CS I)
- Identifisere non-seminomer som morfologisk er seminomer
- Vurdere klassifisering i prognosegrupper ved metastatisk non-seminom
- Diagnostisere germinalcellesvulster i mediastinale eller retroperitoneale masser
- Evaluere behandlingseffekt
- Identifisere tilbakefall
Humant choriogonadotropin type beta (ß-hCG)
ß-hCG er lett/moderat forhøyet avhengig av tumorvolum hos 40–60 % av pasientene med non-seminom og 20–50 % av pasientene med seminom. Høye nivåer av ß-hCG (> 200) bør gi mistanke om non-seminomatøse germinalcelle-komponenter, det vil si koriokarsinom og tumorprøven skal undersøkes på nytt av erfaren patolog. Forekomst av nekrose i primærsvulsten kan forklare hvorfor det kan finnes non-seminomatøse komponenter i metastaser, men ikke i primærsvulsten.
Halveringstiden i serum for ß-hCG skal være ≤ 3 dager. Reduksjonshastigheten i konsentrasjonen av ß-hCG etter kjemoterapi kan imidlertid følge et mer komplekst mønster, med tilsynelatende lengre halveringstid på sene tidspunkter av kjemoterapien, selv hos pasienter der behandlingen er vellykket (Christensen et al., 1999).
Det kan oppstå kryssreaksjon med beta-enheten til LH og gi en falsk positiv test. Dessuten kan hypogonadisme indusere produksjon av LH samt ß-hCG i hypofysen. Kortvarig substitusjonsbehandling med testosteron undertrykker utskillelsen av LH og ß-hCG fra hypofysen, og gjør det mulig å måle ß-hCG med opprinnelse i germinalceller.
ß-hCG kan også produseres av svulster av annen opprinnelse som lever-, bukspyttkjertel-, mage-, nyre- og blærekreft (Kliesch et al., 2003).
Alfaføtoprotein (AFP)
I germinalcellesvulster utskilles AFP av embryonalt karsinom og plommesekktumor. AFP er per definisjon ikke forenlig med en seminomdiagnose. Påvisning av signifikant forhøyede nivåer av AFP hos en pasient med seminom skal derfor medføre at tumorprøven undersøkes på nytt med tanke på non-seminomatøse komponenter. Selv om slike ikke blir funnet, skal svulsten betraktes og behandles som et non-seminom! Man bør være klar over at reparative og infeksiøse/virale prosesser i leveren samt skrumplever og traumer også kan indusere en økning av AFP, enkelte ganger så høyt som > 500 ng/ml. I sjeldne tilfelle kan pasienter konstitusjonelt ha et AFP-nivå som er moderat forhøyet over normalområdet. Et stabilt moderat forhøyet AFP-nivå kan dermed være forenlig med en seminomdiagnose. Halveringstiden i serum for AFP skal være ≤ 7 dager.
AFP kan også være forhøyet ved hepatocellulært karsinom samt ved kreft i bukspyttkjertel, magekreft, kolorektal kreft og bronkiekreft.
Laktatdehydrogenase (LDH)
LDH er et cytoplasmatisk enzym i alle levende celler og det ses forhøyede verdier ved alle typer vevsødeleggelse og celledød. Totalt LDH-nivå i serum er forhøyet hos ca. 40–60 % av pasientene med testikkelkreft av germinalcelletype. Vanligvis er det isoenzym LDH1 som er forhøyet. Forhøyet LDH tas i betraktning i den prognostiske klassifiseringen av risikogruppe for non-seminomer, men er mindre spesifikk for germinalcellesvulster enn AFP eller ß-hCG. Det ses ofte ubetydelig forhøyede LDH-nivåer under oppfølging.
Placentaliknende alkalisk fosfatase (PLAP)
PLAP er forhøyet hos 50 % av pasientene med seminom, men analyseres bare ved noen få laboratorier (Weissbach, Bussar-Maatz, & Mann, 1997). Det kan også være forhøyet ved non-seminomatøse tumorer som inneholder seminom-komponenter. Bruk av denne markøren er valgfri. Den kan spesifikt vurderes i evalueringen av uklare lesjoner, som ved stadium IIA eller restsykdom etter kjemoterapi. Det kan være den eneste forhøyede markøren ved metastatisk sykdom og kan dermed være nyttig under oppfølging av slike pasienter. PLAP kan være falskt forhøyet hos røykere.
MicroRNA (MIR371–374)
Standard tumormarkørene (ß-hCG, AFP, LD) er forhøyet hos færre enn 60 % av alle testikkelkreftpasienter, avhengig av histologi og stadium. Nye markører er derfor på sin plass. MikroRNA-er er for tiden under evaluering som nye biomarkører (Almstrup et al., 2020).
Fertilitetsmålinger og hormonanalyser
Sist faglig oppdatert: 15.04.2021
Kryopreservering av sæd skal tilbys før orkiektomi, opp til 55 år. Det kan være regionale variasjoner når det gjelder aldersgrenser. Hvis det ikke utføres før orkiektomi, skal det alltid tilbys før oppstart av alle typer behandling, selv om adjuvant kjemoterapi mest sannsynlig ikke har noen langvarig skadelig effekt på spermatogenesen (Eberhard et al., 2004; Weibring et al., 2019). Pasienter som gjennomgår flere sykluser med kjemoterapi, strålebehandling eller retroperitoneal lymfeknutedisseksjon (RPLND), har risiko for subfertilitet/infertilitet.
Kjønnshormoner (LH/FSH, testosteron og SHBG) skal analyseres før og etter orkiektomi og under oppfølging. Serumprøver til hormonanalyser skal fortrinnsvis tas om morgenen eller senest før kl. 12 (på bakgrunn av den normale døgnvariasjon).
Alle pasienter som gjennomgår kjemoterapi eller strålebehandling, bør bruke prevensjon i seks måneder etter avsluttet behandling.
Inguinal eksplorasjon, orkiektomi og biopsi
Sist faglig oppdatert: 15.04.2021
Det anvendes et snitt t som ved åpen lyskebrokkoperasjon. Fremre vegg i lyskekanalen åpnes, og ductus deferens og karene i funikkelen dissekeres fri ved indre lyskeåpning. I de fleste tilfeller er diagnosen sikker, og karene i funikkelen og ductus deferens deles umiddelbart. Testis med epididymis og omgivende tunica vaginalis presses ut av skrotum og dissekeres fri fra skrotalveggen. Ductus deferens og karene i funikkelen ligeres og deles separat ved indre lyskeåpning. Venen i funikkelen skal merkes med en sutur for å gjøre fullstendig fjerning av vena testicularis lettere ved en senere RPLND. Urologen skal ikke skjære gjennom preparatet.
Hvis det er tvil om diagnosen, for eksempel ved små svulster, blir funikkelen klemt av før mobilisering og inspeksjon av testis. I noen tilfeller blir tunica albuginea incidert, og det sendes et frysesnitt til histologi. Slike biopsier bør fortrinnsvis gjøres som enukleasjonsbiopsi, slik at selve tumor ikke incideres. Hvis resultatet av frysesnittet er en benign tilstand (for eksempel adenomatoid tumor eller epidermoid cyste), anbefales det å utføre en lokal reseksjon i stedet for en orkiektomi.
Det anbefales å tilby alle pasientene en testikkelprotese før orkiektomi (Skoogh et al., 2011). Selv om kirurgiske komplikasjoner er sjeldne, vil et en del pasienter være utilfreds med størrelsen, formen, plasseringen eller konsistensen til protesen (Dieckmann et al., 2015). Det er svært viktig med rådgivning før operasjonen. Hvis pasienten ønsker å få en testikkelprotese, anbefales det å lukke åpningen til pungen med en absorberbar tobakkspung-sutur over protesen og/eller fiksere protesen, for å forhindre migrasjon i retning opp mot lysken.
Hemiskrotektomi
I sjeldne tilfeller hvor en svulst invaderer skrotumveggen, bør det utføres en hemiskrotektomi. Hvis svulsten har blitt incidert med risiko for at den har gitt lokal utsæd av cancerceller, kan hemiskrotektomi vurderes (Capelouto, Clark, Ransil, & Loughlin, 1995; Khetpal, Katz, Cox, & Arnaoutakis, 2014; Leibovitch, Baniel, Foster, & Donohue, 1995).
Organbevarende kirurgi
Organbevarende kirurgi ved testikkelkreft blir bare indisert i noen få utvalgte tilfeller og anbefales ikke hvis den kontralaterale testikkelen er normal. Hvis radiologene sterkt antyder en mistenkelig benign tumor (f.eks. epidermoid cyste), kan reseksjon av tumoren vurderes, selv ved normal kontralateral testikkel. Hos disse pasientene bør man imidlertid gå videre til radikal orkiektomi hvis frysesnittanalyse avslører en malign tumor. For små tilfeldige svulster som oppdages ved ultralyd, se Tilfeldig funn av mindre lesjoner i testis.
Indikasjoner for organbevarende kirurgi er svulster i begge testikler eller i én solitær testikkel. Målet er å bevare en viss endokrin funksjon. Tumorvolumet bør være mindre enn 30 % av testikkelvolumet. Svulsten skal reseseres med en fri rand av omkringliggende vev. Det bør tas flere biopsier fra kanten/bunnen av operasjonsfeltet samt én eller to tilfeldige biopsier i selve testikkelen for å utelukke utbredt germinalcelleneoplasi in situ (GCNIS). Det har nylig blitt stilt spørsmål ved nødvendigheten av å klemme av blodtilførselen og kjøle testikkelen under prosedyren. Tumorreseksjon er en prosedyre som sjelden blir utført, og bør utføres på sentre med erfaring med å håndtere disse pasientene. Alle pasienter skal tilbys adjuvant lokal strålebehandling på grunn av den høye risikoen (> 85 %) for konkomitant GCNIS. Strålebehandlingen kan forsinkes med de samme forholdsreglene som nevnt i Håndtering av GCNIS. (Albers, Goll, Bierhoff, Schoeneich, & Muller, 1999; Coogan et al., 1998; Harland et al., 1998).
Biopsi av kontralateral testikkel
Pasienter med risikofaktorer anbefales en kontralateral biopsi for å påvise mulig GCNIS, og dette gjøres på tidspunktet for orkiektomien.
Risikofaktorene er i tillegg til en kontralateral germinalcelletumor:
- Kryptorkisme
- Tidligere infertilitet eller spermiekonsentrasjon ≤ 10 mill/ ml
- Atrofisk testikkel (< 12 ml)
- Arv
- Microlithiasis
Hos pasienter over 40 år uten risikofaktorer er risikoen for GCNIS svært lav, og biopsi anbefales ikke.
En prosedyre med dobbeltbiopsi gir økt sensitivitet sammenlignet med en prosedyre med enkeltbiopsi (Dieckmann, Kulejewski, Pichlmeier, & Loy, 2007; Kliesch et al., 2003) og anbefales. Kirurgiske komplikasjoner rapporteres å forekomme hos
2–3 % av pasientene og håndteres for det meste konservativt (Dieckmann, Heinemann, Frey, Pichlmeier, & German Testicular Cancer Study, 2005).
Dobbeltbiopsien utføres best på denne måten:
Testikkelen holdes fast og presses opp mot huden. Det legges et lite skrotalsnitt over testikkelen, og tunica vaginalis åpnes. Tunica albuginea incideres ved øvre pol, lateralt (for å spare hovedblodkaret i testikkelen). Den første biopsien tas på dette stedet. Klipp løs en ren prøve med tubulus med en fin, skarp saks (prøve på 3–4 mm). Det lille snittet i tunica lukkes deretter med en kontinuerlig sutur. Deretter legges det andre snittet i tunica albuginea i nedre pol og klipp ut en tilsvarende prøve som fra øvre pol . Unngå nok en gang midtlinjen for å spare hovedblodkaret. Lukk innsnittene i tunica albuginea og hud hver for seg.
Ved en atrofisk testikkel er én enkelt biopsi tilstrekkelig.
Under utføring av biopseringen er forsiktig håndtering av biopsien og plassering i fikseringsmiddel viktig for å forhindre mekanisk skade. For undersøkelse mtp GCNIS anvendes formalin som fikseringsvæske. Evalueringen av GCNIS i testikkelbiopsier krever erfaring, og den patologiske undersøkelsen av biopsiene bør omfatte immunohistokjemi.
Microlithiasis
Testikulær microlithiasis som eneste risikofaktor er ikke en indikasjon for biopsi (Balawender, Orkisz, & Wisz, 2018).
Hos menn som søker behandling for infertilitet og presenterer seg med testikkelatrofi (én eller begge testikler er under 12 ml) og/eller en historie med kryptorkisme, er testikkelbiopsi indisert hvis det er påvist testikulær microlithiasis.
Håndtering av GCNIS
Sist faglig oppdatert: 15.04.2021
Stråling av testiklene vil føre til utrydding av alle germinalceller og permanent sterilitet. Hvis pasienten har et ønske om fremtidig fertilitet, bør følgende forholdsregler tas:
- Det anbefales kryopreservering av sæd før stråling
- Ved azoospermi (ingen sperm i ejakulatet) og et sterkt ønske om bevaring av fertilitet, flere testikkelbiopsier, og hvis det er påvist intratestikulære forlengede spermatider, er etterfølgende kryopreservering et alternativ som skal diskuteres med pasienten
- Unilateral testikulær germinalcellekreft (TGCT) og GCNIS i kontralaterale testikkel:
- Hvis pasienten har en partner, et umiddelbart ønske om å få barn og god spermkvalitet (dette spørsmålet må diskuteres med en androlog/fertilitetsspesialist), kan det anbefales overvåkning i noen måneder til noen år, mens paret prøver å bli gravide. I løpet av denne perioden bør ultralyd utføres hver sjette måned, og pasienten bør oppfordres til selvundersøkelse.
- Hos pasienter som ikke gjennomgår kjemoterapi: GCNIS kan utryddes ved lokal stråling gitt som 9 daglige doser på 2 Gy (totaldose – 18 Gy). Det har blitt foreslått at stråledosen skal økes til 20 Gy. Så langt finnes det imidlertid ingen evidens for å vise at 20 Gy innebærer en lavere risiko for behandlingssvikt, men synes å være forbundet med en høyere risiko for androgenmangel (Bang et al., 2009). Selv om noen av mennene senere utvikler hypogonadisme, er androgen substitusjon ikke nødvendig hos mer enn 50 %, i hvert fall de første årene etter lokal stråling.
- Hos pasienter som gjennomgår kjemoterapi: Platinaholdig kjemoterapi kan utrydde GCNIS. Pasienter med GCNIS kan utvikle invasiv kreft til tross for kjemoterapi (Kleinschmidt, Dieckmann, Georgiew, Loy, & Weissbach, 2009). Det sikreste alternativet er å gi lokal stråling som angitt under a). Et alternativ er å gjenta biopsien, 1–2 år etter fullført kjemoterapi, og utføre ultralyd hver sjette måned frem til biopsien. Hvis det finnes GCNIS-celler, skal det tilbys stråling. Man må imidlertid være klar over at etter kjemoterapibehandling kan GCNIS-cellene reduseres i antall uten å bli fullstendig utryddet. Det anbefales derfor en dobbelt biopsi ettersom sensitiviteten til én enkelt testikkelbiopsi er forventet å være lavere enn tallene gitt ovenfor, og risikoen for sen kontralateral TGCT er til stede. Selv om den nye biopsien er negativ, bør det utføres ultralyd av testikler én gang i året under oppfølgingen.
- Pasienter med ekstragonadal sykdom og GCNIS i én testikkel: Det anbefales orkiektomi av den rammede testikkelen.
- Bilateral GCNIS: Stråling som angitt under 1b.
- Unilateral GCNIS og ingen malignitet i den andre testikkelen: Orkiektomi.
Prosedyren for strålebehandling av GCNIS finnes her.
Retningslinjer for oppfølging etter testikkelstråling for GCNIS
- Det bør gjøres en dobbel kontrollbiopsi av testikkelen 24 måneder etter stråling og denne skal bare avsløre «Sertoli-only»-mønster. Forekomst av germinalceller indikerer svikt i strålebehandlingen.
- Serumnivåer av testosteron, SHBG, LH, og FSH bør kontrolleres før strålebehandlingen, og 6 og 12 måneder etter. Deretter skal testene gjentas med et intervall på 12–24 måneder.
- Det bør utføres ultralyd av testiklene når oppfølgingen er fullført etter 5 eller 10 år.
Tilfeldig funn av mindre lesjoner i testis
Sist faglig oppdatert: 15.04.2021
Med økende anvendelse av høyfrekvent skrotal ultralyd, har tilfeldig funn av små avgrensede ekkoforandringer i testikkelen dukket opp som et problem i klinisk praksis. Hos pasienter med normale tumormarkører er en betydelig del av disse mindre (< 1 cm) asymptomatiske lesjonene benigne. Svært små svulster (≤ 5 mm) kan obseveres med ultralyd etter 2 måneder. Når histopatologi av lesjonen anses som nødvendig på grunn av vekst, bør dette oppnås ved enukleasjon av svulsten med frysesnittanalyse. Perkutan nålebiopsi bør ikke utføres. Spesifisiteten til frysesnittanalyse i denne situasjonen er > 90 %. Hvis frysesnittet avslører malignitet, anbefales radikal orkiektomi.
Enukleasjonsbiopsi kan assisteres av ultralydveiledning utført av en erfaren radiolog.
Patologisk undersøkelse av testikkel
Sist faglig oppdatert: 15.04.2021
ICCR-retningslinjene for neoplasi av testis og KVAST.
Makroskopiske forhold og snittuttak
- Side, testikkelstørrelse, tumorstørrelse og de makroskopiske egenskapene til svulsten, som makroskopisk involvering av epididymis, funikkel og tunica vaginalis.
Snittuttak: 1 cm2 for hver cm av maksimal tumordiameter, inkludert makroskopisk normalt parenkym (hvis til stede), tunica albuginea og epididymis samt andre mistenkelige områder. Minst ett proksimalt og ett distalt snitt av funikkelen i tillegg til ethvert mistenkelig område.
Mikroskopiske forhold og diagnose
- Histologisk type iht. WHO 2016. Bare rene seminom tumorceller klassifiseres som et seminom. Spermatocytisk tumor er ikke inkludert i denne protokollen.
- Forekomst eller fravær av vaskulær svulstinvasjon, invasjon i stromal rete testis, tunica albuginea, tunica vaginalis, epididymis eller funikkel.
- Forekomst eller fravær av intratubulær germinalcelleneoplasi (ITGCN) i parenkymet som er utenfor selve svulsten.
- pT-kategori iht. TNM 8. versjon.
- Immunohistokjemisk evaluering bør brukes ved diagnostiske problemer.
Stadiebestemmelseundersøkelser
Sist faglig oppdatert: 15.04.2021
Tester som skal utføres etter orkiektomi – kliniske stadiebestemmelseprosedyrer
- Serumnivåer av AFP, ß-hCG, LDH, (PLAP er valgfritt).
- Serumnivåer av LH, FSH, testosteron og SHBG.
- CT thorax, abdomen og bekken med intravenøs og oral kontrast, hvis ikke gjort før orkiektomi. Hvis det er klinisk indikasjon på at avansert metastatisk sykdom foreligger, skal CT gjøres før orkiektomien.
- MR av hjernen er nødvendig hos pasienter med kliniske symptomer eller tegn som indikerer hjernemetastaser, hvis ß-hCG er > 50 000, massive lungemetastaser samt hos pasienter med ikke-pulmonale viscerale metastaser.
- MR av ryggrad og bekken er nødvendig hos pasienter med kliniske symptomer eller tegn på skjelettmetastaser og hos pasienter med ikke-pulmonale viscerale metastaser.
- Andre undersøkelser kan indiseres individuelt.
- Følg tumormarkører ukentlig til nadir/normalisering i henhold til halveringstid. Hos pasienter som er antatt å være i stadium I, eller hvis det er tvil om stadiet, fortsettes det til restadiebestemmelseen er fullført.
- En restadiebestemmelse med CT og tumormarkører, som ovenfor, bør gjøres for alle pasienter som antas å være stadium I-pasienter og har non-seminom 6–8 uker fra orkiektomi.
Eventuelle klare avvik fra plottene for halveringstid indikerer metastatisk sykdom, og dermed avsluttes observasjonsperioden.
PLAP kan også analyseres i seminom og er forbundet med metastatisk sykdom når den er forhøyet hos en ikke-røyker.
Hvis det er symptomer eller tegn til metastatisk sykdom, skal pasienten umiddelbart henvises til en kreftavdeling for videre evaluering og behandling.
Prognostisk gruppeklassifisering ved metastatisk sykdom bør utføres umiddelbart før behandling.
Pasienter med lett forstørrede paraaortale lymfeknuter/ mistenkte metastaser i antatt klinisk stadium IIA med negativ markører
Lett forstørrede retroperitoneale lymfeknuter <2 cm hos pasienter uten forhøyede tumormarkører representerer et diagnostisk problem. Disse lymfeknutene kan være benigne eller representere metastaser. Det anbefales en observasjonsperiode på 8 uker (seminom) /
6–8 uker (non-seminom) med en restadiebestemmelse med mindre metastatisk sykdom bekreftes av en biopsi. Se Metastatisk seminom, avsnitt Seminom i klinisk stadium IIA–IIB (≤ 3 cm), om Kjemoterapi og Metastatisk non-seminom, avsnitt Non-seminom CS IIA Mk-.
Serumnivåer av ß-hCG og AFP må overvåkes annenhver uke i løpet av dette observasjonsintervallet.
Positronemisjonstomografi (PET)-CT kan tilføre informasjon om seminomer, men er ikke nødvendigvis pålitelig når det gjelder mindre lymfeknuter. Selv om en PET-CT er positiv, kan en biopsi vurderes hvis det er mulig.
Hos pasienter med lav-volum metastatisk seminom (< 3 cm i axialplan) er preoperativ PET-CT obligatorisk for alle pasienter som er planlagt for kirurgi.
Hvis stadiet fortsatt er usikkert etter en 6–8 ukers observasjonsperiode, er ytterligere observasjon berettiget, alternativt kan en laparaskopisk lymfeknutebiopsi/-reseksjon være et alternativ. Hvis en biopsi ikke er mulig, kan en unilateral RPLND vurderes og diskuteres på et regionalt eller nasjonalt multidisiplinært teammøte, MDT.
Kjemoterapi (eller strålebehandling) bør ikke initieres med mindre det er sikker metastatisk sykdom (f.eks. vekst eller positiv biopsi).
Bildediagnostikk
Diagnostikk og stadiebestemmelse
Sist faglig oppdatert: 23.05.2025
Ultralyd brukes til å bekrefte en intratestikulær masse, mikroforkalkninger, forekomst av synkrone svulster og til å måle volumet av begge testikler. Andre bildebehandlingsprosedyrer av testiklene bør ikke utføres rutinemessig.
Computertomografi (CT) thorax, abdomen og bekken er nødvendig som en del av primærstadiebestemmelseen. Orale og intravenøse kontrastmedier er obligatorisk ved baseline. Hvis det blir funnet en solitær eller flere små (<5 mm) lungenoduler, må beslutningen om biopsi eller oppfølging tas individuelt for hver pasient (MacMahon et al., 2017).
Ved tolkning av retroperitoneale lymfeknuter ved CT, uavhengig av hvilke størrelseskriterier som brukes for metastaser, bør den begrensede sensitiviteten og spesifisiteten for karakterisering av lymfeknuter vurderes i den kliniske håndteringen (Forsberg et al., 1986; Hale et al., 2018). Derfor er differensieringen mellom de kliniske stadiene I og IIA upålitelig, hvis ß-hCG og AFP er normale. En detaljert beskrivelse av lokalisasjonen, antallet og størrelsen på lymfeknutene, helst i tre dimensjoner, men i det minste med målene på de to lengste perpendikulære aksiale diametrene bør være med i radiologirapporten (Handbook for reporting results of cancer treatment., 1979; Honecker et al., 2018). MR av abdomen og bekken er forbundet med lignende begrensninger i sensitivitet og spesifisitet i stadiebestemmelsesituasjonen (Hale et al., 2018; Sohaib et al., 2009), og har ikke vist seg å gi ytterligere informasjon for denne sykdommen. MR er det foretrukne alternativet hos pasienter der intravenøse kontrastmedier ikke kan gis.
På grunnlag av tilgjengelige data har FDG-PET ikke vist seg å forbedre sensitiviteten for stadiebestemmelse av testikkelkreft sammenlignet med CT-undersøkelse alene (Albers, Bender, et al., 1999; M. de Wit et al., 2008; Huddart et al., 2007; Spermon, De Geus-Oei, Kiemeney, Witjes, & Oyen, 2002). I denne gjeldende prospektive behandlingsprotokollen anbefales PET-skanninger før primær kirurgisk behandling for metastatisk seminom med lavt volum samt metastatisk nonseminom med lavt volum.
MR er den foretrukne metoden for å undersøke forekomsten av hjerne- eller skjelettmetastaser. Se Stadiebestemmelseundersøkelser for indikasjoner. Andre undersøkelser bør utføres i henhold til symptomer
Behandlingsevaluering
Sist faglig oppdatert: 15.04.2021
Standard modalitet for responsevaluering er CT thorax, abdomen og bekken. MR brukes for pasienter med kontraindikasjoner mot CT. En detaljert beskrivelse av lokalisasjonen, antallet og endringer i størrelsen på metastatiske lokalisasjoner med målene på minst de to lengste perpendikulære aksiale diametrene bør være med i radiologirapporten (Handbook for reporting results of cancer treatment., 1979).
Bildestyrt responsevaluering under behandling av metastatisk sykdom er en utfordring. Responsevaluering skal alltid utføres på et sykehus med et tverrfaglig team bestående av radiologer, onkologer og kirurger tilgjengelig, alle med erfaring i behandling av pasienter med germinalcellesvulster.
PET-CT under behandlingen har for tiden ingen bevist nytte utenfor kliniske studier/prospektive protokoller.
For bruk av PET-CT med hensyn til håndtering av tumormasser i seminom etter kjemoterapi, se Metastatisk seminom, avsnitt Restlesjoner etter kjemoterapi ved seminom.
Oppfølging
Sist faglig oppdatert: 15.04.2021
Det er ønskelig å redusere den totale stråledosen fra gjentatte diagnostiske bildebehandlingsprosedyrer for pasienten uten å gå på akkord med kvaliteten på oppfølgingen. Dette er spesielt viktig hos pasienter som er under 35 år ved diagnosetidspunktet. MR av lymfeknuter i abdomen- og bekkenområdene er den foretrukne metoden for å undersøke retroperitoneum under oppfølging.
Ultralyd kan også utføres hvis den nødvendige kompetansen er tilgjengelig. Ultralyd av retroperitoneum er imidlertid vanligvis mindre sensitiv når det gjelder å oppdage retroperitoneale lymfeknuter enn MR eller CT. I tvilstilfeller skal det derfor utføres en MR-undersøkelse. Siden CT er assosiert med uønsket total stråledose for unge pasienter hvis det gjentas mange ganger under oppfølgingen, anbefales det å utføre MR minst én gang årlig hvis det brukes ultralyd i oppfølgingen.
MR utføres i henhold til prinsippene i Bildeprotokollen. Det er nødvendig med dialog med den ansvarlige radiologen for å sikre at prinsippene i protokollen og årsakene til oppfølgingen er forstått fullt ut.
Klinisk stadium I
Seminom
Sist faglig oppdatert: 23.05.2025
Bakgrunn for seminom i klinisk stadium I
Seminom utgjør nær 60 % av pasientene med testikkelkreft. Av disse presenterer 85 % seg med klinisk stadium I, noe som gjør seminom CS I til den vanligste formen for testikkelkreft (28).
Den optimale håndteringen av seminom CS I er fortsatt gjenstand for debatt. I EGCCCG (European Germ cell Cancer Consensus Group) var det ingen konsensus om anbefalt behandling for pasienter med seminom CS I (Beyer et al., 2013). I gjeldende retningslinjer fra ESMO og EAU er både overvåkning og karboplatin anbefalte behandlingstilbud, men adjuvant behandling ble ikke anbefalt for pasienter med lav risiko. Risikoen og fordelene ved ulike tilnærminger må diskuteres med pasienten med tanke på de ulike fordelene og ulempene, både på kort og lang sikt. Manglende etterlevelse av overvåkningsstrategier er fortsatt gjenstand for bekymring.
SWENOTECA-erfaring med seminom CS I
SWENOTECA V-protokollen (2000–2006) var et svensk-norsk program som omfattet pasienter med seminom i alle stadier. Pasienter med CS I kunne velge enten adjuvant strålebehandling 25,2 Gy/14 fraksjoner eller overvåkning. I 2004, da resultatene av den randomiserte studien som sammenlignet adjuvant karboplatin og strålebehandling ble anerkjent, ble alternativene endret slik at de også omfattet adjuvant karboplatin (Tandstad et al., 2011). I SWENOTECA VII-protokollen (2007–2010) ble behandlingen i CS I justert etter de mulige prognostiske risikofaktorene, det vil si størrelsen på primærtumoren og stromainvasjon i rete testis (Warde et al., 2002). Pasienter med 0–1 risikofaktorer ble anbefalt overvåkning, men kunne velge én kur adjuvant karboplatin (AUC7), mens pasienter med to risikofaktorer ble anbefalt én kur adjuvant karboplatin, men kunne velge overvåkning. I 2014 ble det utført en analyse av CS I-pasienter inkludert i SWENOTECA VII frem til 2010 og pasienter fra SWENOTECA V behandlet med adjuvant karboplatin. Totalt 1064 pasienter ble inkludert i analysen, 669 pasienter fikk adjuvant karboplatin, 339 ble administrert ved overvåkning og fire pasienter fikk andre adjuvante behandlinger. Stromainvasjon av rete testis og tumorstørrelse > 4 cm ble begge bekreftet som uavhengige risikofaktorer som predikerer tilbakefall i en multivariat analyse.
Overvåkning
Basert på store, uselekterte pasientserier om overvåkning vet vi at 85 % av pasientene i CS I blir kurert av orkiektomi alene (Daugaard, Petersen, & Rorth, 2003; Kollmannsberger, Tyldesley, et al., 2010; Tandstad et al., 2011). Den totale overlevelsen i disse pasientseriene nærmer seg 100 %, og overvåkning er en attraktiv strategi. Det har vært gjort flere forsøk på å identifisere mulige prognostiske faktorer for tilbakefall. En banebrytende artikkel publisert i 2002 samlet 638 pasienter fra fire sentre (Warde et al., 2002). Denne retrospektive studien identifiserte tumorstørrelse > 4 cm og stromainvasjon i rete testis som uavhengige risikofaktorer for tilbakefall. En upublisert valideringsstudie fra samme gruppe kunne imidlertid ikke bekrefte den prognostiske verdien av disse foreslåtte risikofaktorene (Chung et al., 2010). Resultatene fra SWENOTECA V, publisert i 2011, klarte heller ikke å identifisere noen prognostiske faktorer for tilbakefall (Tandstad et al., 2011). Resultater fra en nyere spansk risikotilpasset protokoll gir noen indikasjoner på at pasienter uten noen av de foreslåtte risikofaktorene har svært lav risiko for tilbakefall (Aparicio et al., 2011). Publiserte resultater fra SWENOTECA VII bekreftet lav tilbakefallsrisiko hos denne pasientgruppen, med en tilbakefallsrate på 4,0 %. Både invasjonen av rete testis og tumorstørrelse > 4 cm ble funnet å være risikofaktorer som predikerer tilbakefall. I SWENOTECA VII var tilbakefallsraten hos pasienter med 1–2 risikofaktorer og administrert med overvåkning 15,5 % (Tandstad et al., 2016).
Adjuvant karboplatin
I 2005 ble det rapportert resultater fra en stor randomisert studie av én kur adjuvant karboplatin versus adjuvant strålebehandling (Oliver et al., 2005). De modne dataene ble presentert i 2011 (Oliver et al., 2011). Studien inkluderte 1447 pasienter med en median oppfølgingstid på 6,5 år. 573 pasienter fikk én kur karboplatin (AUC 7). Tilbakefallsraten etter én kur adjuvant karboplatin var 5,3 %. Kombinerte resultater fra SWENOTECA V og VII, hvor 669 pasienter fikk én kur karboplatin, viste en tilbakefallsrate på 6,2 % etter en median oppfølgingstid på 5,2 år (Tandstad, Cavallin-Stahl, et al., 2014; Tandstad et al., 2011; Tandstad et al., 2016). Stromainvasjon av rete testis eller tumorstørrelse > 4 cm gir en høyere risiko for tilbakefall, 9,4 %. På grunn av disse resultatene ble ABC-studien initiert av SWENOTECA i 2015. Studien randomiserer pasienter med 1–2 risikofaktorer til BEP x 1 eller standard adjuvant behandling med karboplatin. For detaljer om studien se www.swenoteca.org.
Karboplatin har et bratt dose-respons-intervall. Det er rapportert dårligere resultater med flere tilbakefall når det har blitt gitt en lavere dose enn AUC 7. AUC 7 skal alltid beregnes ut fra ukorrigert GFR, målt ved iohexol- eller Cr-EDTA- clearance. eGFR basert på både Cystatin-C og kreatinin kan også brukes, med unntak av pasienter på kortisonmedisiner eller avkreftede pasienter. Se www.egfr.se for ytterligere informasjon og for å beregne ukorrigert/absolutt GFR som skal brukes til dosering av karboplatin.
Flere ikke-randomiserte studier har undersøkt to kurer med adjuvant karboplatin (AUC 7 eller 400 mg/m2), med en rapportert tilbakefallsrate på ca. 2 % (Aparicio et al., 2005; Aparicio et al., 2011; Steiner et al., 2002; Steiner et al., 2011).
Hvis det gis adjuvant kjemoterapi, bør den startes opp snarest mulig etter den definitive kliniske stadiebestemmelseen.
Doseringsplan for Karboplatin.
Adjuvant strålebehandling
Inntil nylig har standard adjuvant behandling av seminom CS I vært strålebehandling. Basert på store randomiserte studier utført av MRC, vet vi at 20 Gy gitt til et paraaortalt felt gir et tilbakefall på ca. 4 % (Oliver et al., 2011). I SWENOTECA V var tilbakefallsraten etter 25,2 Gy til paraaortale og ipsilaterale iliacale lymfeknuter 0,8 % (Tandstad et al., 2011). På grunn av den økte risikoen for hjerte- og karsykdom og sekundære kreftformer etter strålebehandling, anbefales strålebehandling ikke lenger som en standard adjuvant behandling. Strålebehandling kan likevel være et alternativ hos dem som ikke er egnet for adjuvant kjemoterapi eller overvåkning.
Behandlingsanbefalinger ved seminom klinisk stadium I
Både invasjon av rete testis og tumorstørrelse > 4 cm predikerer tilbakefall etter overvåkning eller adjuvant karboplatin (AUC7). Pasienter uten noen av disse foreslåtte risikofaktorene har lav risiko for tilbakefall. SWENOTECA foreslår en modifisert risikotilpasset strategi for adjuvant behandling ved seminom CS I. Pasienten bør gis grundig informasjon både muntlig og skriftlig som tar pasientens autonomi i betraktning.
- Pasienter med tumor ≤ 4 cm og ingen stromal invasjon av rete testis anbefales overvåkning
- Pasienter med tumor > 4 cm og/eller stromal invasjon av rete testis er overvåkning og én kur karboplatin AUC 7 likeverdige alternativer
- Adjuvant strålebehandling anbefales kun til dem som ikke er egnet for adjuvant kjemoterapi eller overvåkning. Se kapittel Strålebehandling for detaljer.
Non-seminom
Sist faglig oppdatert: 15.04.2021
Bakgrunn for non-seminom i klinisk stadium I
Omtrent 60 % av pasientene med non-seminom er i klinisk stadium I. I EGCCCG var det konsensus om å anbefale behandling med adjuvant BEP x 1 til høyrisikopasienter (forekomst av lymfovaskulær invasjon) og overvåkning til lavrisikopasienter (Beyer et al., 2013). Retningslinjene fra ESMO og EAU anbefaler overvåkning og adjuvant BEP som behandlingstilbud basert på forekomst av risikofaktorer. Risikoen og fordelene ved hver strategi bør diskuteres med pasientene med tanke på den umiddelbare og langsiktige påvirkningen, også for lavrisikopasienter. Manglende etterlevelse av overvåkningsstrategier er fortsatt gjenstand for bekymring. RPLND er et alternativ i spesifikke situasjoner.
Risikoen for okkult metastatisk sykdom ved CS I NSGCT er svært avhengig av forekomsten av lymfovaskulær invasjon (LVI) i svulsten (Klepp et al., 1991; Pont et al., 1996). LVI er til stede i omtrent en tredjedel av svulstene. Hos pasienter med svulster med forekomst av LVI, er risikoen for tilbakefall ca. 50 %, og hos de uten LVI, ligger den på 15–20 % (Kollmannsberger, Moore, et al., 2010; Sturgeon et al., 2010). Tilbakefall forekommer oftest i retroperitoneum, og de fleste tilbakefall blir påvist innen 2 år etter orkiektomi (Kollmannsberger et al., 2015; Tandstad, Stahl, et al., 2014). Håndteringsalternativer for CS I NSGCT er overvåkning, retroperitoneal lymfeknutedisseksjon (RPLND) og adjuvant kjemoterapi. Behandlingsbeslutningen bør alltid være basert på en grundig diskusjon med pasienten om fordeler og ulemper ved hver strategi. Det skal også tilbys skriftlig pasientinformasjon
SWENOTECA-erfaring med non-seminom CS I
SWENOTECA har publisert populasjonsbaserte data om risikotilpasset behandling for CS I NSGCT hvor 745 pasienter ble inkludert i perioden 1998–2005. Målet var å redusere risikoen for tilbakefall og dermed redusere behovet for senere «salvage»-kjemoterapi samtidig som høye kurasjonsrater opprettholdes. Pasienter med LVI+ ble behandlet med én kur BEP og pasienter med LVI- kunne velge mellom overvåkning eller én kur BEP. Ved en median oppfølging på 4,7 år reduserte én kur BEP tilbakefallsfrekvensen med 90 % hos pasienter med både LVI+ og LVI-, noe som ga en tilbakefallsrate på henholdsvis 3,2 % og 1,4 %. Det ble publisert en oppdatering i 2014 som inkluderte 517 pasienter behandlet med én kur BEP. Med en median oppfølging på 8 år ble resultatene bekreftet, uten tilbakefall etter 3,3 år og 100 % kreftsspesifikk overlevelse (Tandstad, Stahl, et al., 2014).
Overvåkning
Enkelte sentre går inn for overvåkning for alle CS I NSGCT, følgelig vil ingen pasienter bli behandlet unødvendig. Imidlertid vil 50 % av de med LVI og 15 % av pasientene uten LVI trenge «salvage»-behandling senere (Daugaard et al., 2003; Kollmannsberger, Moore, et al., 2010; Nichols et al., 2013; Sturgeon et al., 2010; Tandstad et al., 2009). Etterlevelse av oppfølgingsplanene er helt avgjørende hvis det brukes overvåkning.
Adjuvant BEP
Én kur BEP reduserte risikoen for tilbakefall med 90–95 % hos alle pasienter (Tandstad, Stahl, et al., 2014).
Primær nervesparende retroperitoneal lymfeknutedisseksjon (RPLND)
Primær nervesparende bilateral RPLND – er standardbehandlingen hos pasienter med CS I med maligne somatiske transformasjoner i testikkelsvulsten (Honecker et al., 2018). Behandling etter RPLND må vurderes i henhold til patologiske funn. Primær unilateral RPLND kan diskuteres som et alternativ til overvåkning eller adjuvant kjemoterapi hos pasienter som ikke er villige til å gjennomgå noen av disse behandlingsstrategiene.
Behandlingsandbefalinger ved non-seminom klinisk stadium I
- En risikotilpasset strategi for adjuvant behandling ved seminom CS I. Pasienten bør gis grundig informasjon muntlig og skriftlig som tar pasientens autonomi i betraktning
- Høyrisikopasienter, LVI+ (lymfovaskulær invasjon), anbefales én kur adjuvant BEP
- Pasienter med LVI- kan velge mellom de likeverdige alternativene overvåkning eller adjuvant BEP
Se unntak nedenfor.
NB:
Pasienter med malign somatisk transformasjon i testikkelsvulsten
- Primær bilateral nervesparende RPLND – er standardbehandlingen hos pasienter med CS I med malign somatisk transformasjon (Honecker et al., 2018). Behandling etter RPLND må vurderes i henhold til patologiske funn (Giannatempo et al., 2016).
Postpubertalt teratom bare i testikkelsvulsten
- Bør følges som non-seminom stadium I overvåkning, og bør ikke få adjuvant behandling
Hvis det gis adjuvant kjemoterapi, bør den startes opp snarest mulig etter den definitive kliniske stadiebestemmelseen. Generelt anbefaler vi ikke adjuvant behandling initiert senere enn tolv uker etter orkiektomi. BEP-regimet blir brukt. Når bleomycin gis på dag 15, bør det tas fullstendig hematologisk staatus for toksisitetsevaluering. SWENOTECA «Behandlingsblankett» fylles ut og registreres i det nasjonale SWENOTECA-kvalitetsregisteret. Registrering av toksisitet er spesielt viktig. Det anbefales tillegg av G-CSF for å minimere risikoen for toksisitet også i den adjuvante situasjonen.
Metastatisk sykdom
Generelle kommentarer om metastatisk sykdom
Sist faglig oppdatert: 15.04.2021
Ved sikker metastatisk sykdom bør kjemoterapi starte snarest mulig etter at stadiebestemmelseen er gjennomført. Ved sykdom med uttalt spredning og livstruende dårlig prognose må orkiektomi ikke forsinke initieringen av kurativ kjemoterapi.
Ved håndtering av metastatisk sykdom påvirker sykdomsstadiet og risikoklassifiseringen i henhold til retningslinjene fra IGCCCG (International Germ Cell Cancer Collaborative Group) retningslinjene for behandling. Den opprinnelige IGCCCG-klassifiseringen fra 1997 er oppdatert, basert på 12 135 pasienter som ble behandlet i perioden 1990–2013 (International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group, 1997). De oppdaterte resultatene støtter prinsippene i den opprinnelige klassifiseringen, men antyder at prognostiseringen kan videreutvikles.
Dosereduksjoner og behandlingsforsinkelser bør unngås. Det anbefales G-CSF som primær profylakse, se G-CSF.
Pasienter bør vurderes for tromboseprofylakse, se Tromboemboliske hendelser, avsnitt om Anbefalinger for tromboseprofylakse.
Pasienter med intermediær og dårlig prognose skal overføres til et senter med erfaring i behandling av avanserte germinalcellesvulster før behandlingsstart.
Behandling av progredierende sykdom skal diskuteres i SWENOTECA-nettverket.
Behandling av metastatisk sykdom hos eldre pasienter må gjøres med stor forsiktighet med tanke på deres individuelle toleranse.
Metastatisk seminom
Sist faglig oppdatert: 23.05.2025
Ved abdominal sykdom med lite volum er unilateral retroperitoneal lymfeknutedisseksjon den foretrukne behandlingen, (RPLND). Alternative behandlinger består av kjemoterapi eller strålebehandling. Pasienter med mer avansert sykdom anbefales behandling med cisplatin i kombinasjon med etoposid og bleomycin (BEP), subsistuert med etoposid og cisplatin (EP) eller med tillegg av ifosfamid (PEI) hos pasienter med kontraindikasjoner mot bleomycin, se Konvensjonell kjemoterapi, avsnitt om Bleomycin, avhengig av prognostisk risikogruppe.
5-års PFS (%) 1997 | 5-års PFS (%) Oppdatering 2020 | 5-års OS (%) 1997 | 5-års OS (%) Oppdatering 2020 | |
|---|---|---|---|---|
| God | 82 | 89 | 86 | 95 |
| Intermediær | 67 | 79 | 72 | 88 |
| Metastatisk seminom: PFS og OS i henhold til IGCCCG-klassifiseringen i den opprinnelige publikasjonen, til en nylig IGCCCG-oppdatering (4902 pasienter behandlet i perioden 1990–2013, inkludert SWENOTECA-data) | ||||
SWENOTECA-erfaring med seminom CS II–IV
Av 102 pasienter med seminom CS IIA/B fikk tre pasienter (2,9 %) tilbakefall. Hos alle tre var primærbehandlingen strålebehandling, og én pasient fikk tilbakefall innenfor strålingsfeltet. Hos 73 pasienter behandlet med kjemoterapi (EP/BEP) ble det ikke rapportert om tilbakefall (Tandstad et al., 2011). Alle de 86 (6 %) pasientene med mer avansert tumorspredning (CS IIC/D, III og IV) ble behandlet med kjemoterapi initialt. Den kreftspesifikke overlevelsen etter 5 år for alle pasienter behandlet for seminom var utmerket med en overlevelse på 99,6 %, med en kreftspesifikk overlevelse etter 5 år for metastatisk seminom med god prognose på 97,2 %.
Utfordringen er å minimere behandling og oppfølging uten å gå på akkord med det onkologiske utfallet for disse unge mennene og å beholde fertiliteten og livskvaliteten. Den observerte overlevelsen i den intermediære prognosegruppen er usikker på grunn av det lave antallet behandlede pasienter, men tiltak bør gjøres for å forbedre overlevelsen hos denne lille pasientgruppen.
Seminom i klinisk stadium IIA–IIB (≤ 3 cm)
Pasienter med seminom CS IIA har en begrenset sykdom med abdominale lymfeknutemetastaser som er < 2 cm i største aksiale diameter. Derfor er tumormarkørene vanligvis negative. For å gjennomføre riktig stadiebestemmelse av disse pasientene, se Stadiebestemmelseundersøkelser, avsnitt om Pasienter med lett forstørrede paraaortale lymfeknuter/ mistenkte metastaser i antatt klinisk stadium IIA med negativ markører.
Standardbehandlingen av CS IIA har vært strålebehandling eller kjemoterapi. Strålebehandling gis til et paraaortalt og ipsilateralt iliacalt felt. En måldose på 25–30 Gy resulterer i en tilbakefallsfri overlevelse på 88–95 %, ifølge data i litteraturen (Classen et al., 2003; Tandstad et al., 2011). Akkumulerte data om alvorlige seneffekter etter strålebehandling har anført kjemoterapi som et alternativ til strålebehandling hos denne pasientgruppen, med svært få tilbakefall (Tandstad et al., 2011). Kjemoterapi fører imidlertid også til langsiktig morbiditet (Hellesnes et al., 2019).
Ettersom seminom hovedsakelig metastaserer lymfatisk, er primær RPLND en overbevisende behandlingsstrategi, med lavere risiko for seneffekter4 (Alexander, White, & Horwich, 2010; Classen et al., 2003; Eberhard et al., 2004; A Horwich, Fossa, Stenning, Bliss, & Hall, 2010; Tandstad et al., 2011; Zagars, Ballo, Lee, & Strom, 2004).
Flere grupper har rapportert resultater etter bruk av RPLND som primærbehandling av seminom, og det har nylig blitt publisert en gjennomgang av emnet (Heidenreich, Paffenholz, Nestler, Pfister, & Daneshmand, 2020; B. Hu & Daneshmand, 2018). Det er kjente risikoer ved RPLND, inkludert retrograd ejakulasjon, infeksjoner, blødning og chyløs ascites. Prosedyren er standardbehandling ved reseksjon av restlesjoner etter kjemoterapi ved metastatisk non-seminom, og i primærbehandling av nonseminom CS IIA Mk-. Kirurgi er mye mer komplisert etter kjemoterapi sammenlignet med primær RPLND, hvor risikoen for dødelighet rapporteres å være nær null (Baniel, Foster, Rowland, Bihrle, & Donohue, 1994; Beck, Peterson, Bihrle, Donohue, & Foster, 2007; Heidenreich et al., 2003). For tiden inkluderer flere studier pasienter for primær RPLND ved «non-bulky» metastatisk seminom, dvs. CS IIA + IIB ≤ 3 cm, 1–2 metastatiske lymfeknuter. Data fra flere tidligere små studier har vist en tilbakefallsrate nær null i denne pasientgruppen, forutsatt at stadiebestemmelseen ble bekreftet kirurgisk (B. Hu, Shah, Shojaei, & Daneshmand, 2015; Mezvrishvili & Managadze, 2006).
Gitt den økende bevisstheten om seneffekter forbundet med kjemoterapi og strålebehandling, og den kjente effekten av RPLND i regional kontroll av seminom, er det rimelig å anbefale RPLND som et alternativ for førstelinjebehandling.
For tiden har vi tre mulige behandlingsalternativer ved «non-bulky» metastatisk seminom, alle med gode kurasjonsrater: primær RPLND, kjemoterapi og strålebehandling.
Primær RPLND
Primær unilateral RPLND anbefales for pasienter med seminom CS IIA–IIB ≤3 cm (noen diameter) med 1–2 forstørrede retroperitoneale lymfeknuter innenfor templat for unilateral RPLND, se (RPLND), avsnitt om RPLND-templater.
Preoperativ FDG-PET CT
Preoperativ FDG-PET CT skal utføres og vil ha kliniske implikasjoner.
Resultat av preoperativ FDG-PET-CT | Kliniske implikasjoner |
|---|---|
Høyere stadium, dvs. mer enn 2 lymfeknuter eller > 3 cm (noen diameter) eller sykdom utenfor unilateralt templat for RPLND | Behandling i henhold til stadium og risikogruppe |
Positiv FDG-PET-CT innenfor unilateralt templat for RPLND | Primær unilateral RPLND |
Negativ FDG-PET | Hvis tumormarkør (ß-hCG / PLAP) positiv – Primær unilateral RPLND Hvis tumormarkør (ß-hCG / PLAP) negativ – Biopsi hvis mulig, hvis seminom bekreftet – Primær unilateral RPLND |
Hvis patologirapporten fra RPLND avslører upstadiebestemmelse av klinisk stadium, dvs. > 2 lymfeknuter med seminom eller enhver lymfeknute med seminom > 3 cm, anbefales 1 adjuvant syklus BEP og diskuteres på regionalt eller nasjonalt multidisiplinært teammøte, MDT.
Den viktigste mulige seneffekten av dette behandlingsalternativet er Retrograd ejakulasjon. Følgelig må alle pasienter tilbys preoperativ kryopreservering av sædceller, hvis dette ikke allerede har blitt utført.
Disse pasientene bør ha en ekstra oppfølgingskontroll med radiologisk vurdering to måneder etter operasjonen.
Kjemoterapi
BEP x 3 er standard kjemoterapiregime. Hvis det er kontraindikasjoner mot bleomycin, se kapittel Konvensjonell kjemoterapi, avsnitt om Bleomycin, skal EP x 4 velges.
Strålebehandling IIA/ IIB
Målvolumet inkluderer paraaortale og ipsilaterale iliacale lymfeknuter til en måldose på 30 Gy med 2,0 Gy per fraksjon x 15, se kapittel Strålebehandling. For CS IIB bør det gis en tilleggsdose (boost) til GTV på 6 Gy som simultan integrert boost (SIB).
Behandlingsanbefalinger for klinisk stadium IIA + IIB ≤ 3 cm seminom, 1–2 metastatiske lymfeknuter
Kirurgi: Primær RPLND (+ adjuvant BEP x 1, hvis patologirapporten avslører upstadiebestemmelse) Kjemoterapi: BEP x 3 (bleomycin kontraindisert: EP x 4) Strålebehandling: 2,0 Gy x 15 til paraaortale og ipsilaterale iliacale lymfeknuter. Flytskjema: Appendix III |
Klinisk stadium IIB (> 3 cm) – seminom IV
I International Germ Cell Consensus Classification klassifiseres metastatisk seminom som god eller intermediær prognose. Negative prognostiske faktorer er ikke-pulmonale viscerale metastaser eller LDH >2,5 x ULN.
CS IIB-pasienter ble tidligere behandlet med strålebehandling. De rapporterte tilbakefallsratene er 9–24 %, basert på små pasientserier (Chung et al., 2004; Classen et al., 2003; Domont et al., 2013; Tandstad et al., 2011). Tilbakefallene etter strålebehandling er hovedsakelig lokalisert utenfor retroperitoneum. I SWENOTECA-pasientserien hadde 67 pasienter med seminom CS IIB behandlet med kjemoterapi en tilbakefallsfri overlevelse på 100 % etter median 5,5 års oppfølging. Selv om det ikke finnes randomiserte studier som sammenligner strålebehandling og kjemoterapi ved CS IIB, anbefales kjemoterapi til pasienter med CS IIB > 3 cm, på grunn av de høye rapporterte tilbakefallsratene med strålebehandling.
For seminomer i høyere stadier enn CS IIB er det internasjonal konsensus om behandling med 3–4 sykluser med cisplatinbasert kombinasjonskjemoterapi (Albers et al., 2011; Beyer et al., 2012). Siden pasienter med avansert seminom er sjeldne, finnes det ingen randomiserte studier som sammenligner ulike cisplatinbaserte kjemoterapiregimer for seminompasienter alene.
I sjeldne tilfeller av rent seminom i testikkelen og ß-hCG >5000 ved starten av kjemoterapi for metastatisk sykdom, bør pasienten behandles som metastatisk non-seminom, intermediær prognose. Denne anbefalingen er basert på klinisk erfaring, ettersom biopsi av metastaser i slike tilfeller noen ganger har avslørt non-seminomatøse komponenter.
Seminom med god prognose, klinisk stadium IIB (> 3 cm) – IV
En randomisert EORTC-studie som sammenlignet BEP x 3 versus EP x 4 for seminomer med god prognose, rapporterte komplett respons på 95 % versus 87 % (p=0,0075) (R. de Wit et al., 2001). Videre mener vi at BEP x 3 har mindre akutte og langsiktige bivirkninger enn EP x 4, på grunn av lavere kumulativ cisplatindose. Standardbehandlingen av metastatiske seminomer med god prognose er derfor BEP x 3. For pasienter med kontraindikasjoner mot bleomycin, se Konvensjonell kjemoterapi, avsnitt om Bleomycin. Det kan gis EP x 4 eller alternativt PEI x 3. Hos pasienter med nedsatt nyrefunksjon kan fire kurer med karboplatin AUC7 dag 1 med standarddose etoposid dag 1–5 være et behandlingsalternativ. Kjemoterapi med karboplatin i standarddosering gitt alene er dårligere enn cisplatin-basert kombinasjonkjemoterapi ved avansert sykdom (Bokemeyer et al., 2004; A. Horwich et al., 2000), men karboplatin AUC10 kan være et alternativ ved alvorlig nedsatt nyrefunksjon (Tookman et al., 2013).
Behandlingsanbefalinger for seminom med god prognose, klinisk stadium IIB (> 3 cm) – IV
Kjemoterapi: BEP x 3 (bleomycin kontraindisert: EP x 4 eller PEI x 3) Flytskjema: Appendix IV |
Kommentarer til seminom med god prognose, klinisk stadium IIB (> 3 cm) – IV
- Evaluering av behandlingseffekt: Etter fullført kjemoterapi, dvs. etter
BEP x 3 / EP x 4 / PEI x 3- Ved restmasse, se avsnitt Restlesjoner etter kjemoterapi ved seminom lengre ned på siden.
- Pasienter med CS IIB med kontraindikasjoner mot kjemoterapi kan behandles med strålebehandling. Hvis det gis strålebehandling, bør det gis en dose på 2 Gy x 15 til en totaldose på 30 Gy til paraaortale og ipsilaterale iliacale lymfeknuter med tillegg av SIB tilsvarende biologisk effektiv dose (BED) 36 Gy til de forstørrede lymfeknutene. Se Strålebehandling.
Seminom med intermediær prognose
Standardbehandlingen for metastatisk seminom med intermediær prognose er BEP x 4 (Honeck er et al., 2018). For pasienter med kontraindikasjoner mot bleomycin, se Konvensjonell kjemoterapi, avsnitt om Bleomycin, er PEI x 4 er den anbefalte behandlingen.
Behandlingsanbefalinger for seminom med intermediær prognose
Kjemoterapi: BEP x 4 (bleomycin kontraindisert, eller hjernemetastase): PEI x 4) Flytskjema: Appendix V |
Kommentarer til seminom med intermediær prognose
- Radiologisk evaluering før 3. syklus
- Ved markørnegativ sykdom og mangel på radiologisk respons etter to kurer med kjemoterapi, bør muligheten for teratom vurderes og det bør utføres en biopsi.
- Ved progredierende sykdom skal pasienten diskuteres i SWENOTECA-nettverket.
- Evaluering av behandlingseffekt: Etter fullført kjemoterapi
- Ved restmasse, se avsnitt under om Restlesjoner etter kjemoterapi ved seminom
Restlesjoner etter kjemoterapi ved seminom
Seminomatøse svulster kjennetegnes ofte av en langsom regresjonsrate etter kjemoterapi. Resttumorer består for det meste av fibrotisk eller nekrotisk vev, men i opptil 30 % av tilfellene inneholdt resttumorene > 3 cm maligne germinalceller (Flechon, Bompas, Biron, & Droz, 2002; Puc et al., 1996; Ravi et al., 1999). Når det gjelder gjenværende seminomlesjoner etter kjemoterapi, har en FDG-PET-undersøkelse en høy negativ prediktiv verdi (95 %) og er utmerket for å utelukke aktiv sykdom i lesjoner ≥ 3 cm (Bachner et al., 2011). Den bør ikke utføres tidligere enn 9 uker etter dag 1 av den siste kuren med cellegift, på grunn av risikoen for falsk positivitet. FDG-PET kan bidra til håndteringen av gjenværende seminomlesjoner, og særlig med tanke på å unngå unødvendig tilleggsbehandling for pasienter med ikke-regredierende lesjoner ≥ 3 cm (De Santis et al., 2004).
Ved gjenværende seminomlesjoner etter kjemoterapi har en FDG-PET lav positiv prediktiv verdi (23 %) for maligne germinalceller. (Cathomas et al., 2018). Dermed bør gjentatt FDG-PET-undersøkelse og biopsi vurderes hvis det er en positiv FDG-PET i denne situasjonen.
- Konsolideringsbehandling etter kjemoterapi (kirurgi eller strålebehandling) bør ikke brukes rutinemessig
- Regredierende eller persisterende restmasse < 3 cm: Overvåk med en hensiktsmessig radiologisk metode (MR, CT) og tumormarkører i serum
- Restmasse ≥ 3 cm og ikke regredierende: FDG-PET-undersøkelse anbefales ikke tidligere enn 9 uker etter dag 1 av den siste kuren med cellegift
- Stabil restmasse og negativ FDG-PET-undersøkelse: Fortsett oppfølgingen
- Stabil restmasse og positiv FDG-PET-undersøkelse: Gjentatt FDG-PET etter 6–8 uker og biopsi før konsolideringsbehandling avgjøres
- Maligne germinalceller: Kirurgi, hvis mulig
- Maligne germinalceller når kirurgi ikke er mulig: Strålebehandling av begrensede felt med en totaldose på 40 Gy i 2 Gy-fraksjoner
Metastatisk non-seminom
Sist faglig oppdatert: 15.04.2021
Kjemoterapi med BEP-regimet er den viktigste førstelinjebehandlingen av metastatisk non-seminom. Hvis det foreligger utilfredsstillende tumormarkørfall (forlenget halveringstid) etter to sykluser med kjemoterapi, anbefales intensivert kjemoterapi. Hvis nivåene i tumormarkører øker under behandlingen (ikke på grunn av «surge» av tumormarkører, dvs. økning av markørverdier de første dagene etter kurstart, på dag 15 i hver kur), må pasienten evalueres på nytt for behandlingsresistente metastaser i hjerne eller skjelett og kontralateral testikkelsvulst, og kirurgi må vurderes for å identifisere hvilke tumorkomponenter som er til stede for å endre behandlingen på en adekvat måte.
Et generelt prinsipp er at pasienter med retroperitoneale lymfeknutemetastaser etter kjemoterapi ≥ 1 cm i største aksiale diameter opereres med RPLND. Det kan imidlertid være unntak, og derfor skal denne beslutningen diskuteres på nasjonale/regionale tverrfaglige møter. Det finnes visse forhold som kan påvirke beslutningen, se Annen kirurgi enn orkiektomi.
Resttumorer utenfor retroperitoneum bør om mulig reseseres på grunn av diskordansratene til retroperitoneale og ekstra-retroperitoneale restmasser (Hartmann, Candelaria, Kuczyk, Schmoll, & Bokemeyer, 1997; Masterson et al., 2012). Hvis patologisk undersøkelse av restvev fra den første lungen viser nekrose, er reseksjon av kontralaterale pulmonale lesjoner ikke obligatorisk (Besse et al., 2009). Man bør imidlertid være klar over muligheten for diskordant histologi, og derfor ha lav terskel for kirurgi i lesjoner som viser tegn på vekst (Schirren et al., 2012).
Flere studier har vist verdien av behandlingsintensivering basert på utilfredsstillende markørfall. SWENOTECA har siden 1995 brukt utilfredsstillende markørfall for å initiere intensivert behandling. Det har blitt vist forbedret overlevelse basert på intensivering i ett trinn under initial behandling (Fizazi, Pagliaro, et al., 2014; Motzer et al., 2007). Ved slutten av den initiale behandlingen kan langsomt fall i tumormarkører være en del av den naturlige kinetikken til tumormarkører, og indikerer derfor ikke behov for intensivering (Christensen et al., 1999). Vi har valgt å endre våre retningslinjer for intensivering til ett trinns intensivering under initial behandling.
Behandlingsanbefalinger for metastatisk non-seminom
Tumormarkører måles ved dag 1, 5 og 15 i hver kur
Responsevaluering av tumormarkører utføres etter 2 kurer, tumormarkørfall bør plottes i grafer, for ß-hCG og AFP
Restvev ≥ 1 cm etter kjemoterapi: Vurder kirurgi på nasjonalt/regionalt tverrfaglig møte
Hvis det finnes annen germinalcellekreft enn teratom i restvev etter kjemoterapi, bør pasienten diskuteres i SWENOTECA eller på nasjonale tverrfaglige teammøter
SWENOTECA-erfaring med non-seminom CS II–IV
I SWENOTECA IV (1995–2010) ble behandlingen av pasienter med metastatisk sykdom styrt av fall i tumormarkører. Alle pasientene fikk initialt 2 kurer med BEP. Senere behandling ble bestemt av fall i tumormarkører. Pasienter med tilfredsstillende markørfall fortsatte med BEP, mens de med utilfredsstillende fall fikk intensivert behandling. Behandlingen ble intensivert i 2 trinn: Første trinn med tillegg av ifosfamid og andre trinn var høydose kjemoterapi med stamcellestøtte (HDCT). Publiserte data fra SWENOTECA inkluderte pasienter behandlet i perioden 1995–2003 (Olofsson et al., 2011). Pasientene med intermediær prognose hadde gunstige resultater sammenlignet med tidligere rapporterte studier (van Dijk, Steyerberg, & Habbema, 2006), og vi anser den individuelle behandlingsintensiveringen basert på forsinket markørfall som en mulig strategi for å unngå over- eller underbehandling av disse pasientene. Av pasientene som var klassifisert som pasienter med dårlig prognose, hadde pasienter med forhøyede nivåer av tumormarkører bare en signifikant bedre OS sammenlignet med pasienter med ikke-pulmonale visceral metastaser (Olofsson et al., 2011).
I SWENOTECA VIII (2011–2020) ble behandlingsanbefalingene ytterligere presisert. Standard førstelinjebehandling var BEP, med unntak av kontraindikasjoner mot bleomycin eller primære metastaser til CNS. Den initiale behandlingen med BEP x 4 i SWENOTECA IV ved god prognose hadde blitt endret til BEP x 3. Prinsippene for behandlingsintensiveringen forble uendret, men intensiveringstrinn 1 ble endret til TIP for pasientene med dårlig prognose med ikke-pulmonale viscerale metastaser eller med mediastinal primær ekstragonadal sykdom.
|
|
5-års PFS (%) 1997 |
5-års PFS (%) Oppdatering 2020 |
5-års OS (%) 1997 |
5-års OS (%) Oppdatering 2020 |
10 år OS (%) SW IV |
|---|---|---|---|---|---|
|
God |
89 |
90 |
92 |
96 |
95 |
|
Intermediær |
75 |
78 |
80 |
89 |
90 |
|
Dårlig |
41 |
54 |
48 |
67 |
67* |
Metastatisk non-seminom: PFS og OS i henhold til IGCCCG-klassifiseringen i den opprinnelige publikasjonen, til en nylig IGCCCG-oppdatering (9530 pasienter behandlet i perioden 1990–2013, inkludert SWENOTECA-data) og resultater fra SWENOTECA IV (behandlet 1995–2003).
* Ekstragonadale tumorer er ikke inkludert
Non-seminomer med god prognose
Non-seminom CS IIA Mk-
Lett forstørrede retroperitoneale lymfeknuter <2 cm hos pasienter uten forhøyede nivåer av tumormarkører representerer et diagnostisk problem. Hvis det er mulig, bør det utføres en biopsi. Hvis biopsien ikke gir noe klart svar eller ikke kan gjennomføres, trengs det videre evaluering for å etablere et «sant» klinisk stadium.
For å redusere risikoen for seneffekter anbefaler SWENOTECA unilateralt templat for RPLND, se (RPLND), med tillegg av adjuvant BEP x 1 hvis det finnes annen germinalcellekreft enn teratom retroperitonealt. Det bør utføres en preoperativ FDG-PET for å samle erfaring fra denne undergruppen av pasienter. Pasienter med rent teratom i testikkelen og metastatisk sykdom med lavt volum kan håndteres med primær RPLND (Foster et al., 1996; Stephenson et al., 2007).
Behandlingsanbefalinger ved non-seminom CS IIA Mk-
Restadiebestemmelse:
Tilbakegang: Som CS I
Voksende lesjoner med normale tumormarkører og negativ biopsi: RPLND. Preoperativ FDG-PET. Hvis retroperitoneal germinalcellekreft (unntatt teratom): Adjuvant BEP x 1
Bekreftet metastatisk sykdom, dvs. stigende tumormarkører og/eller positiv biopsi (unntatt teratom): I henhold til IGCCCG prognosegruppe
Voksende lesjon til > CS IIB med normale tumormarkører: I henhold til IGCCCG god prognosegruppe
Rent teratom i testikkelen: Primær RPLND
Flytskjema: Appendix VI
Disse pasientene bør registreres som sykdom CS IIA Mk- i henhold til primærstadiebestemmelse, uavhengig av ytterligere funn for å muliggjøre identifikasjon i registeret.
Behandlingsanbefalinger for non-seminom med god prognose, ekskludert CS IIA Mk-
Kjemoterapi: BEP x 3 (bleomycin kontraindisert: EP x 4 eller PEI x 3)
Forsinket fall i tumormarkør etter BEP x 2: Intensivering med PEI x 2
Hvis PEI ble gitt initialt: Intensivering med TIP x 2
Kirurgi etter kjemoterapi hvis resttumor er ≥ 1 cm, TM forhøyet og avtagende eller TM lett forhøyet og stabil (såkalt «tail»)
Flytskjema: Appendix VII
Kommentarer til non-seminom med god prognose
En lett forhøyet stabil tumormarkør gir ingen grunn til å fortsette med kjemoterapi utover anbefalingen ovenfor, men heller til å fortsette med reseksjon av gjenværende sykdom.
Radiologisk evaluering med CT thorax, abdomen og bekken bør utføres etter 2 og 3 eller 4 kurer (inkludert pasienter med CS Mk+), for å evaluere tumorregresjon og behovet for kirurgi etter kjemoterapi.
Hvis pasienten har normale tumormarkører før oppstart av kjemoterapi, og radiologisk regresjon etter 2 BEP er mindre enn 25 % (tumorvolum definert som produkter av to perpendikulære aksiale diametere målt med CT), anbefales kirurgi.
Se generelle kommentarer om metastatisk sykdom i Generelle kommentarer om metastatisk sykdom.
Non-seminom med intermediær prognose
Det finnes få kliniske studier på pasienter med intermediær prognose. Den randomiserte fase III EORTC-studien på pasienter med germinalcellekreft med intermediær prognose, sammenlignet paklitaksel-BEP (T-BEP) med standard BEP. Studien ble avsluttet for tidlig på grunn av langsom datainnsamling og OS var ikke statistisk signifikant (R. de Wit et al., 2012).
Behandlingsanbefalinger for non-seminom med intermediær prognose
Behandlingen av pasienter med intermediær prognose er den samme som for pasienter klassifisert som dårlig prognose utelukkende på grunn av forhøyede nivåer av tumormarkører. Se behandlingsanbefaling nedenfor.
Non-seminomer med dårlig prognose
Pasienter med ikke-pulmonale viscerale metastaser, f.eks. hjerne-, skjelett- eller levermetastaser, har ugunstig prognose. SWENOTECA-erfaringen og flere retrospektive analyser har bekreftet at ikke-pulmonale viscerale metastaser, samt primær mediastinal GCT, er forbundet med et dårligere resultat sammenlignet med pasienter som har dårlig prognose utelukkende på grunn av forhøyede nivåer av tumormarkører (N Adra et al., 2014; N. Adra et al., 2016; Feldman et al., 2016; Kollmannsberger et al., 2000; C. Oing et al., 2017).
Det har blitt gjort tallrike forsøk på å forbedre utfallet for pasienter med dårlig prognose ved å intensivere den primære kjemoterapien eller bruke høydose kjemoterapi i tillegg. Mange studier i fase II har rapportert lovende resultater med kureringsrater på 70–75 % (Bhala et al., 2004; Christian et al., 2003; Fizazi et al., 2002; Fossa et al., 2005; Germa-Lluch et al., 1999; Hartmann et al., 2007; Huddart et al., 2015; Schmoll et al., 2003; Tryakin et al., 2011).
Det finnes noen få randomiserte studier (Culine et al., 2008; Daugaard et al., 2010; Fizazi, Delva, et al., 2014; Hinton et al., 2003; Motzer et al., 2007), hvorav tre bruker høydose kjemoterapi med stamcellestøtte i den eksperimentelle armen. Ingen av de få randomiserte studiene av intensivert initial behandling hos pasienter med dårlig prognose har vist noen nytte av intensivering av primærbehandlingen sammenlignet med standard BEP.
I studien som ble rapportert av Motzer, hadde pasienter med forsinket fall i tumormarkører (TM) under initial kjemoterapi en signifikant fordel av høydose kjemoterapi, men ikke pasienter med tilfredsstillende fall i TM. Den nyere studien GETUG-13 er en randomisert studie basert på TM-kinetikk. Pasienter med forsinket fall i TM etter syklus 1 BEP, ble randomisert mellom 3 ytterligere sykluser med BEP og intensivert behandling, inkludert oxaliplatin, paklitaksel og ifosfamid. Studien bekreftet den prognostiske verdien av TM-kinetikk. Dette er den første randomiserte studien som støtter SWENOTECA-prinsippet om å ta TM-kinetikk i betraktning i behandlingsstrategien. SWENOTECA anser studien som bevis på prinsippet, uten nødvendigvis å definere det optimale intensiveringsregimet. I SWENOTECA X anbefaler vi en ett-trinns behandlingsintensivering hos pasienter med forsinket fall i TM-nivåer, sammenlignet med tidligere totrinns intensivering.
Behandlingsanbefalinger for non-seminom med dårlig prognose
Intermediær og dårlig prognose utelukkende basert på forhøyede nivåer av tumormarkører
Kjemoterapi: BEP x 4 (bleomycin kontraindisert: PEI x 4)
Forsinket fall i TM etter BEP x 2: Intensivering med PEI x 3 og stamcellehøsting
Hvis PEI gitt primært: Intensivering med TIP x 3
Kirurgi etter kjemoterapi hvis resttumor er ≥ 1 cm, TM forhøyet og avtagende eller TM lett forhøyet og stabil (såkalt «tail»)
Flytskjema: Appendix VIII
Dårlig prognose med ikke-pulmonale viscerale metastaser eller mediastinal ekstragonadal sykdom
Kjemoterapi: BEP x 4
Hjernemetastaser eller kontraindikasjon mot bleomycin: PEI x 4
Forsinket fall i TM etter BEP x 2: Intensivering med TIP x 1, stamcellehøsting og 2 høydose Karboplatin Etoposid med autolog stamcellestøtte
Kirurgi etter kjemoterapi hvis resttumor er ≥ 1 cm, TM forhøyet og avtagende eller TM lett forhøyet og stabil (såkalt «tail»)
Flytskjema: Appendix IX
Kommentarer til behandling av non-seminom med dårlig prognose
- Pasientene med dårlig prognose representerer en liten andel av metastatisk NSGCT, ca. 15 %, hvorav ca. 60 % har ikke-pulmonale viscerale metastaser og 40 % har dårlig prognose utelukkende på grunn av forhøyede TM-nivåer. De krever behandling på sentre med lang erfaring innen avansert metastatisk germinalcellekreft. Komplekse behandlingsbeslutninger tas etter diskusjon i SWENOTECA-nettverket ettersom den kliniske presentasjonen er variabel og dette behandlingsprogrammet ikke kan dekke alle situasjoner.
- Noen pasienter befinner seg i en alvorlig tilstand når de diagnostiseres, for eksempel lungesvikt på grunn av omfattende lungemetastaser, og starten på kjemoterapi bør ikke forsinkes av orkiektomi hvis diagnosen er sikker. Orkiektomi må utføres senere uten å forsinke den systemiske behandlingen.
- Ved forekomst av massive lunge- eller hjernemetastaser, spesielt hos pasienter med koriokarsinom, kan det oppstå lunge- eller hjerneblødning under initial behandling, og behandlingssenteret bør være forberedt på å håndtere situasjonen. Pasienter må kanskje behandles på intensivavdelingen.
- Tumorlysesyndrom kan forekomme (svært sjelden), og behandlingssenteret bør være forberedt på dette og vurdere profylakse hos pasienter med stor tumorbyrde.
- Pasienter som i utgangspunktet anses som uegnet for oppstart av fulldose kjemoterapi, kan behandles med et innledende 3-dagers regime, etterfulgt av en fulldosesyklus 2 på dag 15.
- Planer for mulig stamcellehøsting og høydose kjemoterapi bør gjøres så tidlig som mulig for å redusere risikoen for forsinkelse av behandlingen.
- I løpet av de første 10–15 dagene etter starten av kjemoterapi kan TM øke (= «surge»). Hvis denne økningen i TM ikke erkjennes, vil fallet i TM feilaktig bli vurdert som forsinket. Derfor bør beregningen av fallet i TM baseres på TM-nivåene på dag 14/15 i den første BEP-syklusen og dag 1, 5 og 14/15 i den andre BEP-syklusen.
- Hos noen pasienter, for det meste hos de med svært høyt TM-nivå før kjemoterapi (ß-hCG > 100 000 IE / L, AFP > 50 000ug / L), kan fallraten for TM være tilfredsstillende etter de første 2 eller 3 kurene med kjemoterapi, men så avtar fallet i TM etter tredje eller fjerde kur for å holde seg lett forhøyet, ofte rundt 30–50, og synker bare sakte. Dette fenomenet (stabile, lett forhøyede markører, såkalt «tail»), skal ikke betraktes som en behandlingssvikt. Hvis man er i tvil, kan man diskuter dette i SWENOTECA-nettverket.
- Pasienter med viabel germinalcellekreft ved residual reseksjon etter kjemoterapi bør diskuteres i SWENOTECA-nettverket angående videre kirurgi og/eller kjemoterapi. Målet bør alltid være å oppnå fullstendig reseksjon av restmasser (Fizazi et al., 2008).
- Se generelle kommentarer om metastatisk sykdom Generelle kommentarer om metastatisk sykdom.
Hjernemetastaser
Sist faglig oppdatert: 15.04.2021
Det er rapportert hjernemetastaser hos 1–2 % av testikkelkreftpasientene og ca. 10 % av pasientene med avansert metastatisk sykdom (Nonomura et al., 2009; Oechsle & Bokemeyer, 2011; Patel et al., 2019). Det mangler prospektive kliniske studier, dataene er fra retrospektive serier. Primær cerebral germinalcellekreft er ikke inkludert i dette programmet.
Det bør vurderes multimodale behandlingsstrategier hos pasienter med hjernemetastaser, inkludert kjemoterapi, muligens høydosebehandling, samt lokal behandling med strålebehandling eller kirurgi. Sekvenseringen av modaliteter er ikke forhåndsbestemt og kan variere i henhold til symptomer, tumorlokalisasjon, multiplisitet og størrelse, målet for behandlingen, dvs. kurativ eller palliativ intensjon. Hvis hjernemetastasen er resektabel, og det er mulig med en kort forsinkelse av oppstarten av kjemoterapi, bør primær hjernekirurgi vurderes (Fossa, Bokemeyer, et al., 1999).
Generelt er kjemoterapi den initiale behandlingen, ettersom de fleste av disse pasientene også har ekstracerebrale metastaser. Det anbefales kjemoterapi som inneholder ifosfamid, på grunn av bedre penetrasjon til CNS (Jacus et al., 2016). Hvis det oppnås komplett remisjon (CR) med primær kjemoterapi, er konsolidering ikke nødvendig (Feldman et al., 2016). Ved progressive metastaser til CNS under kjemoterapi bør det vurderes umiddelbar kirurgi eller strålebehandling. Hvis reseserte rester i hjernen etter kjemoterapi inneholder annen germinalcellekreft enn teratom, bør videre behandling diskuteres i SWENOTECA-nettverket.
Ved hjernemetastaser ved tilbakefall kan HDCT forbedre overlevelsen (Feldman et al., 2016).
Strålebehandling kan gis som helhjernestråling (WBRT) eller fokalt med stereotaktisk strålebehandling (SRT). Det optimale regimet er ukjent. SRT anbefales ved få (maks 4) og mindre metastaser (Sawrie et al., 2008). WBRT kan være bedre enn SRT for å håndtere mikrometastaser. BED > 50 Gy til tumor anbefales dersom WBRT er bestemt på grunnlag av: 1,8 Gy per fraksjon i 22 fraksjoner til en totaldose på 39,6 Gy med samtidig integrert tumorboost med 2,45 Gy per fraksjon i 22 fraksjoner til en totaldose på 53,9 Gy for germinalcellekreft med hjernemetastaser.
Multimodal behandling med kjemoterapi og helhjernestråling kan forårsake progredierende multifokal leukoencefalopati (PML) (Doyle & Einhorn, 2008).
Hos pasienter som får WBRT, forventes det kognitiv toksisitet på lang sikt. Behandling med memantin kan forhindre kognitiv dysfunksjon (Brown et al., 2013).
Behandlingsanbefalinger for hjernemetastaser
Hjernemetastase ved diagnose, se flytskjema: Appendix XI
Førstelinje kjemoterapi: PEI-regime
Hjernemetastase ved tilbakefall, se flytskjema: Appendix XII
Skjelettmetastaser
Sist faglig oppdatert: 15.04.2021
Primære skjelettmetastaser er sjeldne, i SWENOTECA IV er det bare rapportert 3/94 pasienter med dårlig prognose med skjelett-involvering (Olofsson et al., 2011). Prognosen er dyster for skjelettmetastaser ved non-seminom, med rapportert 2-års PFS og OS på henholdsvis bare 24 % og 36 % (C Oing et al., 2014). Det er fortsatt uklart hvilken rolle lokalbehandling har etter optimal systemisk behandling. Når det er mulig, bør det vurderes histologisk undersøkelse av skjelettmetastaser etter kjemoterapi. Benreseksjon er et behandlingsalternativ hvis det blir funnet teratom (Nini et al., 2018). Rollen til ytterligere strålebehandling er ikke klar (C. Oing et al., 2017). Også lokalbehandling som vertebroplastikk og termisk ablasjon er (Gennaro, Sconfienza, Ambrogi, Boveri, & Lanza, 2019) mulige behandlingsalternativer. Disse pasientene bør diskuteres i SWENOTECA-nettverket.
Kjemoterapi
Konvensjonell kjemoterapi
Sist faglig oppdatert: 15.04.2021
Kjemoterapi bør gis uten dosereduksjoner med intervaller på 21 dager. Dosereduksjoner frarådes på det sterkeste. Utsettelse av behandling, maksimalt 3 dager, bør bare skje unntaksvis. Kjemoterapi doseres ut fra kroppsoverflaten (Griggs et al., 2012), med unntak av høydose kjemoterapi.
Cisplatin
Det er avgjørende med hydrering for å forhindre cisplatin-indusert nefrotoksisitet. Kortvarig hydrering med lavt volum administrert poliklinisk er trygt og effektivt. Det anbefales saltvannsinfusjon alene (Crona et al., 2017; Leu & Baribeault, 2010). Cisplatin skal ikke gis hvis GFR < 40 ml/min/1,73 m2 (normalområde 80–125 for aldersgruppen 18–50 år). Hvis GFR derimot reduseres på grunn av tumorobstruksjon og forbedres etter at nyreobstruksjonen er behandlet (f.eks. nefrostomi, stent), skal cisplatin gis i full dose uten dosereduksjon.
Etoposid
Mer enn 90 % av etoposid bindes til plasmaproteiner. Risikoen for myelotoksisitet øker med økt ubundet fraksjon av etoposid, for eksempel som i hypoalbuminemi (Hartmann & Lipp, 2006). Pasienter med albuminnivåer under 35 g/l har en økning i ubundet etoposid, og mer uttalt toksisitet. Dette bør tas i betraktning hos pasienter med hypoalbuminemi. Clearance av etoposid synes å være lavere hos pasienter over 65 år, derfor må det tas hensyn til deres individuelle toleranse ved behandling hos eldre pasienter (Joel, Shah, Clark, & Slevin, 1996).
Bleomycin
Det må tas hensyn til den mulige risikoen for bleomycinindusert pneumonitt (BIP) (Haugnes, Oldenburg, & Bremnes, 2015) og det må vurderes andre regimer hos pasienter med risiko for BIP.
Bleomycin gis ikke til pasienter med:
- Nedsatt nyrefunksjon (eGFR<50) siden det påvirker elimineringen av bleomycin
- Nedsatt lungefunksjon (lungesykdom, storrøyker)
- En kumulativ dose > 300 000 enheter siden det er forbundet med økt toksisitet (O'Sullivan et al., 2003)
En annen risikofaktor for BIP er alder (> 50 år).
Symptomer kan oppstå uker til måneder etter behandlingsstart.
Behandling av bleomycinindusert pneumonitt
BIP er en alvorlig komplikasjon, og pasienter med BIP bør fortrinnsvis diskuteres i SWENOTECA-nettverket. Behandlingen av BIP inkluderer steroider (f.eks. 0,5–1 mg prednisolon/kg/dag). Det kreves profylakse mot pneumocystis med trimetoprim-sulfa hvis det startes høydose steroider.
Imatinib kan forhindre ytterligere pneumonitt og utvikling av fibrose og bør vurderes tidlig i håndteringen av alvorlig BIP. Det anbefales en dose på 300 mg per dag. Når behandlingen er initiert og hvis pasienten responderer, bør behandlingsvarigheten være 3–6 måneder (Langberg et al., 2018). Det kliniske forløpet av BIP bør evalueres ved hjelp av lungefunksjonstester, for eksempel DLCO/CO-diffusjon.
Bleomycin og anestesi
Det er vist en negativ effekt av høyt inspirerte oksygenfraksjoner i løpet av dager eller uker etter eksponering for bleomycin. Det er imidlertid ingen sikker evidens for at oksygennivået har stor betydning for lungekomplikasjoner under/etter operasjon hos pasienter som har blitt behandlet med bleomycin på grunn av metastatisk germinalcellekreft (Sleijfer, 2001). En annen mulig mekanisme for postoperativ BIP er væskeoverbelastning. Derfor er perioperativ oksygenbegrensning hos pasienter som tidligere ble behandlet med bleomycin, ikke nødvendig. Oksygenkonsentrasjonen under operasjonen skal imidlertid holdes på lavest mulig nivå som gir tilstrekkelig oksygenering (gjennomsnittlig 40 % fraksjon av inspirert oksygen) og væskebalansen må overvåkes nøye (Donat & Levy, 1998).
Bleomycin og dykking
Omfattende klinisk erfaring hos pasienter som gjenopptar dykking etter bleomycinholdig kjemoterapi, kombinert med data fra kirurgi hos disse pasientene, konkluderer med at gjenopptakelse av dykking 6–12 måneder etter ukomplisert behandling med 3–4 kurer med bleomycinholdig kjemoterapi vurderes som trygt (R. de Wit et al., 2007).
Høydose kjemoterapi med stamcellestøtte
Sist faglig oppdatert: 15.04.2021
Høydose kjemoterapi (HDCT) har vært brukt hos utvalgte pasienter med germinalcellekreft i mer enn to tiår. Likevel er det fortsatt ingen klar konsensus om valg av pasienter for HDCT og fordelen sammenlignet med konvensjonelt dosert kjemoterapi (CDCT) (Lorch et al., 2011). Selv om flere fase I/II- og retrospektive studier har indikert en mulig rolle for høydosekonseptet, har tre randomiserte studier ikke kunnet vise noen signifikant overlevelsesfordel for HDCT vs. CDCT i primærbehandling (Daugaard et al., 2010; Droz et al., 2007; Motzer et al., 2007).
Det finnes mange studier som støtter overlegenheten til HDCT i forhold til CDCT som «savage»-behandling, inkludert data fra en stor internasjonal database (Lorch, Mollevi, Kramar, Einhorn, & Necchi, 2010). Den eneste prospektive fase III-studien som sammenlignet HDCT med CDCT, viste imidlertid ingen overlevelsesfordel med HDCT-strategien (Pico et al., 2005), men denne studien har blitt kritisert for flere metodiske problemer.
SWENOTECA-erfaringen med HDCT
I perioden september 1995 til juni 2007 ble 55 pasienter behandlet med HDCT i henhold til SWENOTECA IV (Haugnes et al., 2012). SWENOTECA IV brukte to forskjellige høydosesykluser basert på karboplatin/cyklofosfamid i kombinasjon med enten etoposid eller tiotepa. Det ble valgt ut tre pasientgrupper for HDCT: A) utilstrekkelig respons på standarddose intensivert kjemoterapi (BEP samt ifosfamid, n=36), B) funn av vital kreft ved kirurgi etter intensivert kjemoterapi (n=7), C) tilbakefall etter intensivert kjemoterapi (n=12). I situasjon A og C ble det anbefalt to HDCT-sykluser, og i situasjon B én HDCT-syklus. Totalt 27/36 (75 %) og 4/12 (33 %) pasienter fikk begge de tiltenkte HDCT-syklusene i situasjon A og C.
Totaloverlevelse etter median 7,5 års oppfølging var henholdsvis 72 %, 100 % og 58 % i pasientgruppene A, B og C, mens progresjonsfri overlevelse var henholdsvis 64 %, 71 % og 42 %. Tre menn (5,5 %) døde under høydosebehandling ved tre forskjellige institusjoner, alle behandlet før år 2000. Nefrotoksisitet var den vanligste ikke-hematologiske toksisiteten av grad 4, og rammet fem menn (9,1 %). Hematologisk toksisitet var ikke mer uttalt i løpet av den andre kontra den første HDCT-syklusen. Tidsintervallet mellom syklus én og syklus to var median 55 dager (range 30–84). Tid innlagt var median 23 dager (range 12–54) i begge syklusene. Restitusjonstiden var median 10 dager (område 8–17) for nøytrofile i begge sykluser, og 11 dager (område 6–35) for blodplater i begge syklusene.
I behandlingsprogramet SWENOTECA VIII ble høydoseregimet endret til to sykluser karboplatin og etoposid. Siden 2011 og frem til i dag har mer enn 50 pasienter blitt behandlet med dette regimet. Foreløpige data viser at 63 % av pasientene fikk begge syklusene, noe som tyder på at det er mulig med en dublett av kombinasjonen karboplatin-etoposid.
HDCT-regimer
Karboplatin og etoposid er ryggraden i de fleste høydoseregimer. Noen studier har også inkorporert cyklofosfamid eller ifosfamid. Det er uttrykt bekymring for økt risiko for utvikling av sekundær akutt leukemi etter høydose etoposid. Flere studier har vist at risikoen er akseptabelt lav, med en kumulativ insidens i området 1,4 %–2,6 % for kumulative doser på mer enn 2 g/m2 (N. Adra et al., 2016; Houck, Abonour, Vance, & Einhorn, 2004; Wierecky et al., 2005).
Én enkelt HDCT-syklus er sannsynligvis utilstrekkelig for å gi optimalt celledrap, som indikert av resultatene fra en «salvage»-studie (Pico et al., 2005). Derfor anbefaler de fleste studier to HDCT-sykluser. Noen studier har inkludert en triplett av HDCT, men disse studiene har brukt intermediær dosering av de aktive forbindelsene (Feldman et al., 2010; Lotz et al., 2005). Basert på litteraturen og vår egen erfaring anbefaler vi to sykluser karboplatin og etoposid som beskrevet nedenfor. Den andre syklusen er planlagt å starte så snart pasienten har kommet seg etter den første, vanligvis innen 6–8 uker fra oppstart av første CE.
Vi anbefaler et modifisert Einhorn-regime, som er regimet med de beste resultatene og det som brukes mest i dag (N. Adra et al., 2017).
Behandlingsanbefalinger for høydose kjemoterapi
To kurer karboplatin og etoposid, se Appendix XIII
Karboplatin: 8x (absolutt GFR+25) mg på dag -6, -5, -4 og -3 før infusjon av stamceller (dag 0 = minst 72 timer etter avsluttet infusjon av kjemoterapi), til en totaldose på 32AUC. MAKSIMUMDOSE 1085 mg per dag
Absolutt GFR skal brukes til dosering, basert på clearance av iohexol eller clearance av CrEDTA Det maksimale nivået av absolutt GFR som skal brukes, er 130 ml/min (konkordans med en kroppsoverflate på 2,4)
Etoposid: 560 mg/m2 på dag -6, -5, -4 og -3 før infusjon av stamceller (dag 0 = minst 72 timer etter avsluttet infusjon av kjemoterapi), til en totaldose på 2240/m2. MAKSIMUMDOSE 1340 mg per dag
Se høydose kjemoterapiregime (CE) for ytterligere forholdsregler ved dosering og GFR-måling
Stamcellehøsting og praktiske overveielser
PEI- og TIP-regimene mobiliserer stamceller effektivt når de etterfølges av granulocyttkolonistimulerende faktor (G-CSF), filgrastim, som starter 24 timer etter avsluttet kjemoterapi og fortsetter til høstingen er fullført. Standarddosen av G-CSF (filgrastim) er 10 μg/kg for høsting for å forbedre utfallet. Pegylert G-CSF anbefales ikke på grunn av mangel på data om mobiliseringseffekten.
En praktisk tilnærming er å starte kjemoterapi (PEI eller TIP) på en torsdag, og så starte CD34-telling på en mandag eller tirsdag (dag 12 eller 13 etter oppstart av kjemoterapi). Høstingen utføres vanligvis mellom 8–13 dager etter den siste dagen med kjemoterapi. Hvis utstrømningen av CD34+ progenitorceller blir forsinket, vil det være minst tre arbeidsdager igjen å høste på før helgen. Kjemoterapien kan imidlertid ikke forsinkes i en klinisk hastesituasjon.
Vi anbefaler at det høstes minst 7x106 CD34+/kg til to høydosebehandlinger. Celledosen kan ha innvirkning på aplasiperioden, og en høyere dose kan forkorte den kritiske perioden med vanskelige komplikasjoner. Den nedre dosegrensen for én autolog stamcellestøtte er imidlertid 2x106 CD34+/kg, og dermed er > 4x106 CD34 +/kg minimumet som skal høstes.
Hos pasienter med utilstrekkelig innhøsting etter kjemoterapi er mobiliseringsmiddelet plerixafor (Mozobil®) et alternativ (Hamid et al., 2019). Dette stoffet er en CXCR4-antagonist som hjelper stamcellene til å løsne fra benmargen slik at CD34+-cellene går over til blodbanen. Filgrastim må brukes samtidig.
G-CSF
Sist faglig oppdatert: 15.04.2021
Både betydningen av doseintensitet ved avansert sykdom og den etablerte risikoen for sykehusinnleggelse på grunn av febril nøytropeni etter BEP, uavhengig av behandlingsindikasjon, har ført til økt bruk av G-CSF som primærprofylakse.
Det er bekymring for at samtidig bruk av G-CSF øker risikoen for BIP hos eldre pasienter, en observasjon gjort hos pasienter behandlet for Hodgkins lymfom (> 45 år) (Martin et al., 2005). Data om pasienter med germinalcellekreft støtter ikke en slik økt risiko (Fossa et al., 1998; Iwamoto et al., 2018; Saxman, Nichols, & Einhorn, 1997). Primær profylakse med G-CSF anbefales hos pasienter med disseminert sykdom siden doseintensiteten er vesentlig. I en adjuvant setting anbefales det primærprofylakse med G-CS for å redusere risikoen for febril nøytropeni. Hos eldre pasienter (> 50 år) bør mulig risiko for lungetoksisitet med bleomycin tas i betraktning, også med hensyn til bruk av G-CSF.
Tromboemboliske hendelser
Sist faglig oppdatert: 15.04.2021
Omtrent 8–20 % av menn som gjennomgår behandling for germinalcellekreft, diagnostiseres med tromboemboliske hendelser, spesielt venøse, i løpet av eller kort tid etter cisplatinbasert kjemoterapi (Bezan et al., 2017; Paffenholz et al., 2019; Srikanthan et al., 2015; Weijl et al., 2000). Ved germinalcellekreft er de identifiserte risikofaktorene for tromboemboliske hendelser under kjemoterapi abdominalt stadium C–D, forhøyet LDH og sentralvenøs tilgang.
Litteraturen på dette området er sparsom (Gizzi et al., 2016), men tromboseprofylakse bør vurderes når risikofaktorene i anbefalingsboksen nedenfor er til stede. Tromboseprofylaksen bør fortsette til pasienten ikke har tegn på gjenværende germinalcellekreft. Vi anbefaler lavmolekylært heparin (Lyman, Bohlke, Falanga, & American Society of Clinical, 2015), alternativt en direktevirkende oral antikoagulant hos pasienter med lav risiko for trombocytopeni (Farge et al., 2019; Key et al., 2020).
På grunn av risikoen for tromboembolisme med sentralvenøs tilgang, anbefales perifer venøs tilgang på det sterkeste.
Data fra den norske «Studien på testikkelkreft og aerob- og styrketrening» (TAST) tyder på at pasienter som gjennomgår kjemoterapi for testikkelkreft, bør unngå aerob trening med høy intensitet for å unngå å øke risikoen for tromboemboliske hendelser (Thorsen, 2020).
Anbefalinger for tromboseprofylakse
Tromboseprofylakse bør vurderes hos pasienter med noen av følgende risikofaktorer
Sentralvenøs tilgang (perifer venøs tilgang anbefales på det sterkeste)
Abdominalstadium C og D
Metastaser som obstruerer blodkar
Intermediær eller dårlig prognosegruppe
Tidligere tromboembolisk hendelse
Alder > 60 år
Immobilisering/sykehusinnleggelse
Større kirurgi siste 2 uker
Komorbiditet, som alvorlig infeksjon, kardiovaskulær sykdom, nyresykdom, lungesykdom, diabetes
Annen kirurgi enn orkiektomi
(RPLND)
Sist faglig oppdatert: 15.04.2021
Tverrfaglig tilnærming er obligatorisk, og alle pasienter registreres i vår prospektive populasjonsbaserte studie RETROP. De kirurgiske prosedyrene skal utføres på sentre med adekvat kombinert kompetanse og erfaring med behandling av germinalcellekreft og kirurgi etter kjemoterapi.
Indikasjoner
Det anbefales en primær RPLND hos pasienter med vedvarende markørnegativ non-seminom CS IIA, se flytskjema: Appendix VII, hos pasienter med rent teratom i testikkelen og metastatisk sykdom med lavt volum (Foster et al., 1996; Stephenson et al., 2007), og hos pasienter med CS I med maligne somatiske transformasjoner (Honecker et al., 2018).
Primær RPLND er et foretrukket alternativ for seminom CS IIA-B med 1–2 lymfeknuter med 10–30 mm som største aksial diameter, både i primær setting og etter tilbakefall i CS I etter overvåkning eller adjuvant karboplatin (B. Hu & Daneshmand, 2018; Pierorazio & Biles, 2019), flytskjema: Appendix IV.
Da ingen ikke-invasive metoder, inkludert PET, eller prognostiske modeller kan predikere histologi eller differensiering i restvev, er RPLND av gjenværende tumorvev etter kjemoterapi (største transversale diameter ≥ 10 mm, normalisering av markør) obligatorisk for non-seminom (Ehrlich, Brames, Beck, Foster, & Einhorn, 2010; Fizazi et al., 2001; Fossa, Ous, Lien, & Stenwig, 1989; Kollmannsberger, Daneshmand, et al., 2010; Risk & Foster, 2011).
Ved gjenværende lesjoner < 10 mm (største transversale diameter) etter kjemoterapi, er en PC-RPLND ikke obligatorisk. Det er imidlertid fare for gjenværende kreft eller teratom. En PC‑RPLND kan vurderes hvis 1) det var forekomst av teratom i den primære histologien, 2) det er lav grad av krymping, eller 3) det er en cystisk komponent i restmassen i område 1–8.
RPLND-templater
Før templatene blir bestemt, er det viktig å evaluere radiologien både før og etter kjemoterapi og sørge for at alle områder med forstørrede lymfeknuter blir eksidert (Cho et al., 2017; Heidenreich, Paffenholz, Nestler, & Pfister, 2018).
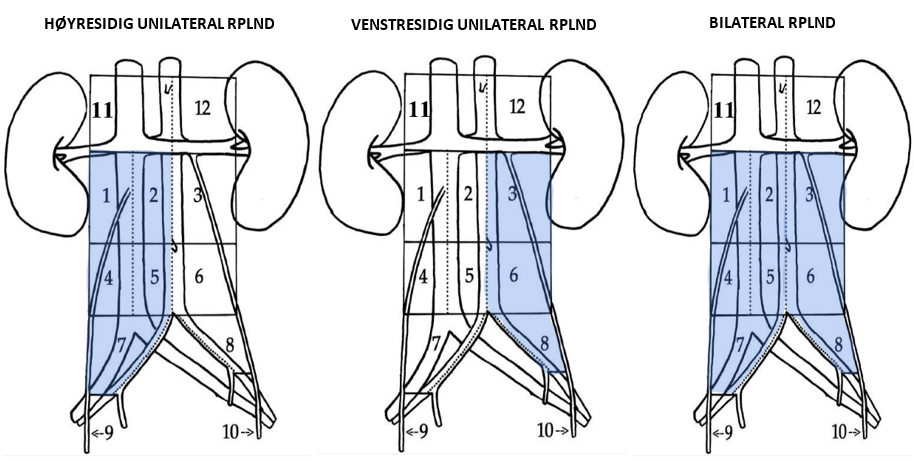
Høyre unilateralt templat
Område 1, 2, 4, 5, 7 og 9
Ved forekomst av en radiologisk synlig masse i område 3 eller 6 bør bilateralt templat benyttes. tillegg
Venstre unilateralt templat
Område 3, 6, 8 og 10
Ved forekomst av en radiologisk synlig masse i område 2 ELLER 5, bør både 2 OG 5 reseseres i tillegg
Bilateralt templat
Område 1–8 OG 9 (høyresidig primærtumor) ELLER 10 (venstresidig primærtumor)
Kirurgisk tilnærming
Sist faglig oppdatert: 15.04.2021
Primær RPLND
For primær RPLND anbefales det en unilateral reseksjon. Det er viktig med en nervesparende tilnærming for å redusere risikoen for retrograd ejakulasjon (Heidenreich et al., 2018; Heidenreich, Pfister, Witthuhn, Thuer, & Albers, 2009).
PC-RPLND for non-seminom
I PC-RPLND for non-seminom med resttumormasse 10–49 mm er et minimums templat en unilateral reseksjon. En lumpektomi (fjerning av bare resttumor) er utilstrekkelig. PC-RPLND skal utføres innen 4–6 uker etter at kjemoterapien er fullført (Masterson & Cary, 2018).
Det anbefales bilateral reseksjon hos pasienter med resttumormasser på ≥ 50 mm og for retroperitoneale ekstragonadale germinalcellesvulster. Suprahilare reseksjoner bør utføres i utvalgte tilfeller med suprahilar resttumor (Heidenreich et al., 2009; Risk & Foster, 2011).
Etter fullført BEP-induksjonskjemoterapi er ca. 11 % av resttumorer viabel cancer, 45 % modent teratom og 44 % nekrotisk eller fibrotisk vev (Gerdtsson et al., 2019).
RPLND har tradisjonelt blitt gjort som konvensjonell «åpen» kirurgi. Imidlertid er minimalt invasiv RPLND et mulig alternativ hos utvalgte pasienter (Cheney, Andrews, Leibovich, & Castle, 2015; Pearce et al., 2017; Stepanian, Patel, & Porter, 2016).
PC-RPLND (seminom)
I motsetning til non-seminom, blir restvev av seminomer etter kjemoterapi vanligvis ikke resektert. Det har blitt brukt en nedre grense 30 mm (største aksiale diameter) for å vurdere biopsi eller reseksjon (hvis mulig) (Herr et al., 1997b). Tidligere retrospektive serier har vist rater for viabel malignitet på 13–42 % i veldefinerte restmasser >30 mm sammenlignet med 0–3 % i masser <30 mm, og kirurgi er teknisk krevende på grunn av fibrose og desmoplastisk reaksjon, og kirurgien viser seg ofte ufullstendig (26–42 %) og forbundet med økt morbiditet. Hvis kirurgi er nødvendig i denne situasjonen bør det være i form av reseksjon (hvis mulig) eller biopsi på grunn av en positiv PET-CT (Daneshmand et al., 2012; Flechon et al., 2002; Ghodoussipour & Daneshmand, 2020; Herr et al., 1997b; Hofmockel, Gruss, & Theiss, 1996; Mosharafa et al., 2003; Puc et al., 1996; Ravi et al., 1999).
Patologiresultater
Sist faglig oppdatert: 15.04.2021
Patologiresultater bør rapporteres i henhold til den patologiske undersøkelsen av lymfeknuter og RETROP-protokollen.
I nyere rapporter har det blitt foreslått at det totale antallet lymfeknuter er en uavhengig prediktor for tilbakefall av sykdom etter PC-PPLND (Carver et al., 2010; Thompson et al., 2010). Patologirapporten bør derfor ikke bare inkludere histologi, men også antall lymfeknuter.
Retrograd ejakulasjon
Sist faglig oppdatert: 15.04.2021
Med en nervesparende tilnærming er det mulig å redusere risikoen for retrograd ejakulasjon. Dette er viktig å tilstrebe ved primær RPLND, og en nervesparende teknikk kan bevare antegrad ejakulasjon hos de fleste pasienter (Donohue & Foster, 1998; Hiester et al., 2018; Risk & Foster, 2011). Nervesparing kan utføres ved spesifikk utdisseksjon av postganglionære nervefibre fra den sympatiske grensestrengen eller ved templatdisseksjon der man unngår å skade nerver som forløper i de regionene av retroperitoneum som ikke dissekeres (se figur for templatfelt). Førstnevnte teknikk er lettest å anvende ved primær RPLND og gir best resultat.
I PC-RPLND anbefales det å forsøke å utføre en nervesparende teknikk, men det er teknisk mulig i bare 20–50 % av tilfellene på grunn av fibrose. Det er viktig er at nervesparende teknikk ikke går på bekostning av radikaliteten. Ejakulasjon bevares hos 68–85 % og 25–45 % av pasientene, som gjennomgår henholdsvis modifisert unilateral eller full bilateral PC-RPLND (Gerdtsson et al., 2019; Luz et al., 2010; Pettus, Carver, Masterson, Stasi, & Sheinfeld, 2009).
Ekstra-retroperitoneale reseksjoner
Sist faglig oppdatert: 15.04.2021
Pasienter med flere lokalisasjoner med gjenværende sykdom etter kjemoterapi, krever for det meste reseksjon av resttumores også utenfor retroperitoneum på grunn av diskordansratene til retroperitoneale og ekstra-retroperitoneale restmasser (Hartmann et al., 1997; Masterson et al., 2012). Rekkefølgen på reseksjonen bør avgjøres på individuell basis og er hovedsakelig avhengig av størrelsen på resttumorene. Store restmasser bør reseseres først på grunn av risikoen for vekst og komplikasjoner (Albers, Melchior, & Muller, 2003).
Resttumorer utenfor retroperitoneum bør reseseres om mulig. Hvis patologisk undersøkelse av restvev fra den første lungen viser nekrose, er reseksjon av kontralaterale pulmonale lesjoner ikke obligatorisk (Besse et al., 2009). Man bør imidlertid være klar over muligheten for diskordant histologi, og derfor ha lav terskel for kirurgi i lesjoner som viser tegn på vekst (Schirren et al., 2012).
På grunn av en høy grad av viabel cancer og teratom etter kjemoterapi, kan kirurgisk reseksjon være nødvendig også for andre metastatiske lokalisasjoner (f.eks. lever, hjerne, hals).
For teratomer er kirurgisk reseksjon den eneste effektive behandlingen.
Kirurgi ved tilbakefall og behandlingssvikt
Sist faglig oppdatert: 15.04.2021
Non-seminom
Markørnegativ (Mk-) og sene residiv
Markørnegative tilbakefall er primært kandidater for kirurgisk reseksjon på grunn av høy risiko for teratom. Annen germinalcellekreft enn teratom vil bli behandlet som markørpositivt tilbakefall, mens pasienter med teratomer vil bli fulgt uten videre behandling.
Ved sene residiv (> 2 år etter initial behandling etterfulgt av fullstendig remisjon), regnes kirurgi som den viktigste delen av behandlingen, og øker kureringsraten (George et al., 2003; Gerl et al., 1997; Oldenburg, Wahlqvist, & Fossa, 2009).
Hos pasienter som tidligere har blitt operert med reseksjon med templat, vil fokuset være på fullstendig fjerning av synlige tumorendringer.
Pasienter som ikke har blitt operert tidligere, bør opereres med unilateral eller bilateral reseksjon avhengig av tumorlokalisasjon.
Kirurgi etter kjemoterapi i andre eller senere linje
Om mulig bør alt gjenstående tumorvev etter kjemoterapi i andre eller senere linje fjernes kirurgisk. Det å oppnå kirurgisk radikalitet og vevshistologi er sterke prediktorer for overlevelse (Fox et al., 1993; Spiess et al., 2006). Prognosen svekkes betydelig svekket ved påvist udifferensiert tumorvev.
Ved økende tumormarkører etter «salvage»-kjemoterapi og mangel på alternativer når det gjelder kjemoterapi (kjemorefraktær sykdom), kan kirurgisk reseksjon av svulster (såkalt «desperation surgery») være et alternativ, forutsatt at komplett reseksjon anses som mulig (Eastham, Wilson, Russell, Ahlering, & Skinner, 1994; Fossa, Stenning, et al., 1999; Murphy et al., 1993; Nichols, 1999). Langtidsoverlevelse kan da oppnås hos inntil 25 % av pasientene. Prognosen er best hos pasienter med sent residiv, moderat forhøyet AFP og begrenset metastatisk lokalisering. Kirurgi har ingen plass ved raskt progredierende sykdom med forhøyet hCG.
Ved flere restteratomer (f.eks. lunge) og plutselig økning i tumormarkør(er) (mistanke om transformasjon til annen germinalcellekreft enn teratom): En PET-undersøkelse og, hvis negativ, ekspektants med ny CT/MR etter 4–6 uker anbefales for å identifisere mulig lokalisasjon for tumorvekst. Dette vil være verdifull veiledende informasjon med tanke på kirurgisk reseksjon.
Seminom
Pasienter med gjenværende sykdom etter andrelinje kjemoterapi
Hos pasienter med sene residiv etter initial kjemoterapi for metastatisk sykdom, er kirurgi som del av «salvage»-behandlingen av største betydning, og alle gjenværende lesjoner bør om mulig fjernes kirurgisk. Disse pasientene bør diskuteres innenfor SWENOTECA-nettverket. Behandlingen bør individualiseres basert på tumorstørrelse, lokalisasjon og funn ved bildediagnostikk. Kirurgi, strålebehandling samt tredjelinje kjemoterapi kan betraktes som en del av behandlingsstrategien.
Kirurgi ved sene residiv
Sene residiv, definert som tilbakefall av sykdom mer enn 2 år etter vellykket initial behandling, er betydelig mindre vanlig ved seminom sammenlignet med non-seminom (Daneshmand et al., 2012). Retroperitonum er den dominerende lokalisasjonen for tilbakefall (Flechon et al., 2002; Oldenburg, Lorch, & Fossa, 2011; Sharp et al., 2008). Behandlingen må individualiseres, basert på tumorstørrelse, funn på PET-bilder og lokalisasjon.
Ved sene residiv med dårlig respons på kjemoterapi bør kirurgisk reseksjon være en viktig del av behandlingsstrategien (Baniel et al., 1995; Dieckmann, Albers, et al., 2005; George et al., 2003; Oldenburg et al., 2011; Oldenburg et al., 2009; Sharp et al., 2008).
Kirurgiske overveielser
Sist faglig oppdatert: 15.04.2021
To typer restmasser etter kjemoterapi er beskrevet og bør identifiseres før operasjonen (Daneshmand et al., 2012; Herr et al., 1997a; Mosharafa et al., 2003; Quek et al., 2005; Ravi et al., 1999). Den ene er den resektable veldefinerte restmassen, som respekterer de omkringliggende strukturene og har blitt rapportert å ha en høyere forekomst av annen germinalcellekreft enn teratom og mindre komplikasjoner. Den andre er den dårlig definerte «plakken» rundt de store blodkarene og ligner retroperitoneal fibrose. Siden disse pasientene for det meste har negative patologiske funn og høy risiko for ytterligere intraoperative prosedyrer (inkludert kompleks vaskulær rekonstruksjon), bør de ikke være kandidater for kirurgi. For ikke-resektable svulster er alternativene stråling eller kjemoterapi, hvis gjenværende malign sykdom er histologisk bekreftet. Det anbefales observasjon hvis biopsier er negative.
Det anbefales å foreta en fullstendig reseksjon av veldefinerte masser (dvs. lumpektomi) når det er mulig, i kombinasjon med tilfeldige åpne biopsier for å sikre en fullstendig evaluering av retroperitoneum (Herr et al., 1997a; Puc et al., 1996; Quek et al., 2005). Biopsier alene er ikke tilstrekkelige til å identifisere restsykdom (Schultz, Einhorn, Conces, Williams, & Loehrer, 1989) (Sakaguchi & Isowa, 2012).
Ekstragonadal germinalcellekreft (EGCT)
Sist faglig oppdatert: 15.04.2021
Av all germinalcellekreft er 2–5 % EGCT (Schmoll, 2002; Stang et al., 2012; Trama et al., 2012). Histologien ligner testikkelkreft, men svulsten oppstår utenfor testiklene, ofte i midtlinjestrukturer fra hjernen til korsbeinet og oppfattes som en egen entitet (Utz & Buscemi, 1971). Mye tyder på at EGCT oppstår i primordiale germinalceller som ikke har fullført migrasjonen fra plommesekken via baktarmen til gonadefolden under organogenesen i fosterlivet. Dette avspeiler også den typiske lokalisasjonen i corpus pineale, mediastinum, retroperitoneum, blære, korsbein og prostata. Vanligste lokalisasjon er fremre mediastinum etterfulgt av retroperitoneum. EGCT utgjør ca. 15 % av alle svulster i fremre mediastinum hos voksne (Ueno et al., 2004).
Ekstragonadal germinalcellekreft i sentralnervesystemet dekkes ikke av dette behandlingsprogrammet.
EGCT skiller seg fra testikulær germinalcellekreft ved en generelt større tumorbyrde, hyppigere lokalisasjon i mediastinum og en større andel av non-seminomer (75–80 %). Det finnes ingen evidens for arvelighet, men det er en assosiasjon til Klinefelters syndrom. Omtrent 1 av 17 pasienter med mediastinal EGCT er rammet av maligne blodsykdommer, som akutt myelogen leukemi og myelodysplastisk syndrom (Hartmann et al., 2000). Disse maligne blodsykdommene er ikke forårsaket av behandling, og har en svært dårlig prognose. Den samme kromosomaberrasjonen som er sett ved testikulær germinalcellekreft (12p), er ofte til stede i leukemiske blastceller (Ueno et al., 2004). Median tid fra diagnose av EGCT til diagnose av disse maligne blodsykdommene er seks måneder.
Klassifisering og prognose
Sist faglig oppdatert: 15.04.2021
Den histopatologiske inndelingen i seminomer og non-seminomer er felles for gonadale GCT og EGCT. Den avspeiler ulike modningstrinn i en normal embryogenese (McKenney, Heerema-McKenney, & Rouse, 2007; Schneider et al., 2001). I en metaanalyse av 635 pasienter med EGCT hadde 83 % non-seminom (197).
Prognosen for EGCT følger IGCCCG-kriteriene, og mediastinal non-seminomatøs EGCT klassifiseres alltid som dårlig prognosegruppe. 54 % ble lokalisert i mediastinum og 45 % i retroperitoneum (Bokemeyer et al., 2002). Median alder ved diagnose var 28 og 30 år for henholdsvis mediastinalt og retroperitonealt non-seminom. Metastatisk sykdom var til stede ved diagnose hos henholdsvis 50 % av pasientene med primær mediastinal EGCT og hos 76 % av pasientene med retroperitoneal tumor.
Diagnostikk
Sist faglig oppdatert: 15.04.2021
Se Appendix X.
Siden det ikke er noen primær testikkelsvulst, diagnostiseres de fleste pasienter på bakgrunn av symptomer fra voksende tumormasser i mediastinum eller retroperitoneum. Skillet mellom en primær testikkelsvulst og EGCT har implikasjoner både for behandling og prognose, og den diagnostiske stadiebestemmelseen bør være grundig for å avsløre mulig patologi i testis.
Begge testiklene bør vurderes med ultralydundersøkelse for å avsløre mulig patologi. I tillegg til en åpenbar primærtumor i testiklene, kan et patologisk ultralydresultat inkludere tegn på tilbakedannet, såkalt «utbrent» tumor («burned-out tumor»). Bilateral biopsi anbefales hos alle pasienter. Dette bør imidlertid ikke forsinke behandlingsstart og kan utføres to år etter avsluttet kjemoterapi. Biopsier utføres både på grunn av muligheten for en uoppdaget primærtumor, og forekomst av GCNIS som kan føre til senere risiko for metakron testikkelkreft, som har blitt rapportert å være ca. 10 % (Hartmann et al., 2001; Hashimoto et al., 2012). Tidligere kunne mange EGCT-svulster, spesielt retroperitoneal EGCT, faktisk ha sin opprinnelse fra en primær testikkelkreft, noe som delvis forklarer den bedre prognosen for retroperitoneal EGCT sammenlignet med EGCT i andre lokalisasjoner (Bokemeyer et al., 2003; Scholz et al., 2002). Hvis det er klinisk mistanke om Klinefelters syndrom hos pasienter med mediastinale svulster, bør kromosomanalyse vurderes.
Det er nødvendig med tumorbiopsi før behandlingsstart med mindre det er forhøyede tumormarkører, eller pasienten befinner seg i en livstruende tilstand.
Behandling
Sist faglig oppdatert: 15.04.2021
Kjemoterapi
For alle EGCT bør flytskjemaet for behandling for den respektive prognosegruppen brukes. Primær non-seminom mediastinal EGCT klassifiseres og behandles som testikkelkreft med dårlig prognose med ikke-pulmonal visceral metastase. Pasienter med EGCT bør kun behandles av et senter med erfaring med avanserte germinalcellesvulster. Teratomer behandles kun ved kirurgisk reseksjon, og kjemoterapi har ingen rolle med mindre det er forhøyede tumormarkører.
De fleste studier på metastatiske non-seminom tumorer med dårlig prognose har inkludert mediastinal EGCT. Standardbehandlingen av alle pasienter med dårlig prognose har vært BEP x 4. Ved non-seminomatøs mediastinal EGCT har det imidlertid blitt anbefalt kjemoterapi med PEI eller VIP for å unngå Bleomycin, ettersom disse pasientene vil kreve omfattende thoraxkirurgi for restmasser etter kjemoterapi (Albany & Einhorn, 2013; Kesler et al., 2008). Kombinasjonen av Bleomycin og thoraxkirurgi øker risikoen for å utvikle bleomycinindusert pneumonitt (Haugnes et al., 2009), se Konvensjonell kjemoterapi. Som med testikulær germinalcellekreft viser data at en gunstig nedgang i serumsvulstmarkører sterkt predikerer forbedrede behandlingsresultater ved EGCT. Det finnes data som indikerer en fordel med behandlingsintensivering ved forsinket fall i tumormarkører hos pasienter med dårlig prognose, inkludert EGCT (Motzer et al., 2007).
For seminom EGCT i den gode prognosegruppen er behandlingsanbefalingen BEP x 3 eller EP x 4. For seminom EGCT i den intermediære prognosegruppen er behandlingsanbefalingen BEP x 4 eller PEI x 4.
Siden SWENOTECA-protokollene inntil nylig ikke har inkludert EGCT, mangler vi egne data for å støtte behandlingen av denne gruppen basert på tumormarkørkinetikk. SWENOTECA VIII-retningslinjene for pasienter i den dårlige prognosegruppen med ikke-pulmonale viscerale metastaser anbefaler behandlingsintensivering sammenlignet med den tidligere SWENOTECA IV-protokollen. Vi velger derfor å behandle EGCT med dårlig prognose som testikkelkreft med ikke-pulmonale viscerale metastaser med dårlig prognose.
Kirurgi
Hos pasienter med non-seminomatøs EGCT oppstått fra mediastinum er kirurgisk reseksjon av alle restmasser etter kjemoterapi obligatorisk og bør utføres uten forsinkelse etter kjemoterapi.
Den gjenværende mediastinale svulsten er ofte stor, og det finnes ofte andre restmasser. Histologiske analyser av kirurgisk fjernede restmasser hos disse pasientene har avdekket annen germinalcellekreft enn teratom hos 66 % av pasientene og teratom hos 22 % (Vuky et al., 2001). Oftere enn ved andre germinalcellesvulster finnes det teratom med malign transformasjon i restmasser. Dette er en entitet med spesielt dårlig prognose, og hvor kirurgi er den eneste sjansen for helbredelse. Hvis fullstendig reseksjon er teknisk mulig, synes kirurgi å være gunstig selv hos pasienter med forhøyede tumormarkører.
Hos pasienter med non-seminomatøs EGCT som oppstår fra retroperitoneum, har reseksjon av restmasser vist annen germinalcellekreft enn teratom hos 25 % av pasientene og teratom hos 16 % av pasientene. Dette er en høyere andel enn hos pasienter med restmasser etter behandling av gonadal metastatisk sykdom. En fullstendig bilateral RPLND er derfor berettiget hos disse pasientene.
Det er ikke sikkert at seminomatøs EGCT krever kirurgi etter kjemoterapi (Albany & Einhorn, 2013; Albany, Kesler, & Cary, 2019; Busch, Seidel, & Zengerling, 2016).
Indikasjonene for adjuvant kjemoterapi ved andre typer germinalcellekreft enn teratom ved restlesjoner hos pasienter i intermediær og dårlig prognosegruppe er usikre, da det ikke finnes sterke evidensbaserte data. Både adjuvant kjemoterapi umiddelbart etter kirurgi og tett overvåkning kan være alternativer. Det viktigste fokuset bør imidlertid være å oppnå radikal kirurgi av alle restlesjoner.
«Salvage»-behandling
«Salvage»-behandling av retroperitoneal EGCT følger anbefalingene for gonadal germinalcellekreft. For mediastinale non-seminomatøse tumorer fører «salvage»-kjemoterapi til langsiktig remisjon hos mindre enn 10 % av pasientene, og derfor bør man vurdere kirurgisk reseksjon selv om tumormarkørene er forhøyet (Albany & Einhorn, 2013; Bokemeyer et al., 2002; Busch et al., 2016).
Registrering og oppfølging
Det er et eget registreringsskjema for pasienter med ekstragonadal germinalcellekreft, men de andre skjemaene er de samme for primær testikulær og primær ekstragonadal lokalisasjon av svulsten. Oppfølgingsplanen er den samme som for metastatisk germinalcellekreft. Ved mediastinal EGCT bør bildediagnostikk med MR eller CT av mediastinum planlegges samtidig som bildediagnostikk av retroperitoneum.
Klassifisering og prognose
Sist faglig oppdatert: 15.04.2021
Den histopatologiske inndelingen i seminomer og non-seminomer er felles for gonadale GCT og EGCT. Den avspeiler ulike modningstrinn i en normal embryogenese (McKenney, Heerema-McKenney, & Rouse, 2007; Schneider et al., 2001). I en metaanalyse av 635 pasienter med EGCT hadde 83 % non-seminom (197).
Prognosen for EGCT følger IGCCCG-kriteriene, og mediastinal non-seminomatøs EGCT klassifiseres alltid som dårlig prognosegruppe. 54 % ble lokalisert i mediastinum og 45 % i retroperitoneum (Bokemeyer et al., 2002). Median alder ved diagnose var 28 og 30 år for henholdsvis mediastinalt og retroperitonealt non-seminom. Metastatisk sykdom var til stede ved diagnose hos henholdsvis 50 % av pasientene med primær mediastinal EGCT og hos 76 % av pasientene med retroperitoneal tumor.
Diagnostikk
Sist faglig oppdatert: 15.04.2021
Se Appendix X.
Siden det ikke er noen primær testikkelsvulst, diagnostiseres de fleste pasienter på bakgrunn av symptomer fra voksende tumormasser i mediastinum eller retroperitoneum. Skillet mellom en primær testikkelsvulst og EGCT har implikasjoner både for behandling og prognose, og den diagnostiske stadiebestemmelseen bør være grundig for å avsløre mulig patologi i testis.
Begge testiklene bør vurderes med ultralydundersøkelse for å avsløre mulig patologi. I tillegg til en åpenbar primærtumor i testiklene, kan et patologisk ultralydresultat inkludere tegn på tilbakedannet, såkalt «utbrent» tumor («burned-out tumor»). Bilateral biopsi anbefales hos alle pasienter. Dette bør imidlertid ikke forsinke behandlingsstart og kan utføres to år etter avsluttet kjemoterapi. Biopsier utføres både på grunn av muligheten for en uoppdaget primærtumor, og forekomst av GCNIS som kan føre til senere risiko for metakron testikkelkreft, som har blitt rapportert å være ca. 10 % (Hartmann et al., 2001; Hashimoto et al., 2012). Tidligere kunne mange EGCT-svulster, spesielt retroperitoneal EGCT, faktisk ha sin opprinnelse fra en primær testikkelkreft, noe som delvis forklarer den bedre prognosen for retroperitoneal EGCT sammenlignet med EGCT i andre lokalisasjoner (Bokemeyer et al., 2003; Scholz et al., 2002). Hvis det er klinisk mistanke om Klinefelters syndrom hos pasienter med mediastinale svulster, bør kromosomanalyse vurderes.
Det er nødvendig med tumorbiopsi før behandlingsstart med mindre det er forhøyede tumormarkører, eller pasienten befinner seg i en livstruende tilstand.
Behandling
Sist faglig oppdatert: 15.04.2021
Kjemoterapi
For alle EGCT bør flytskjemaet for behandling for den respektive prognosegruppen brukes. Primær non-seminom mediastinal EGCT klassifiseres og behandles som testikkelkreft med dårlig prognose med ikke-pulmonal visceral metastase. Pasienter med EGCT bør kun behandles av et senter med erfaring med avanserte germinalcellesvulster. Teratomer behandles kun ved kirurgisk reseksjon, og kjemoterapi har ingen rolle med mindre det er forhøyede tumormarkører.
De fleste studier på metastatiske non-seminom tumorer med dårlig prognose har inkludert mediastinal EGCT. Standardbehandlingen av alle pasienter med dårlig prognose har vært BEP x 4. Ved non-seminomatøs mediastinal EGCT har det imidlertid blitt anbefalt kjemoterapi med PEI eller VIP for å unngå Bleomycin, ettersom disse pasientene vil kreve omfattende thoraxkirurgi for restmasser etter kjemoterapi (Albany & Einhorn, 2013; Kesler et al., 2008). Kombinasjonen av Bleomycin og thoraxkirurgi øker risikoen for å utvikle bleomycinindusert pneumonitt (Haugnes et al., 2009), se Konvensjonell kjemoterapi. Som med testikulær germinalcellekreft viser data at en gunstig nedgang i serumsvulstmarkører sterkt predikerer forbedrede behandlingsresultater ved EGCT. Det finnes data som indikerer en fordel med behandlingsintensivering ved forsinket fall i tumormarkører hos pasienter med dårlig prognose, inkludert EGCT (Motzer et al., 2007).
For seminom EGCT i den gode prognosegruppen er behandlingsanbefalingen BEP x 3 eller EP x 4. For seminom EGCT i den intermediære prognosegruppen er behandlingsanbefalingen BEP x 4 eller PEI x 4.
Siden SWENOTECA-protokollene inntil nylig ikke har inkludert EGCT, mangler vi egne data for å støtte behandlingen av denne gruppen basert på tumormarkørkinetikk. SWENOTECA VIII-retningslinjene for pasienter i den dårlige prognosegruppen med ikke-pulmonale viscerale metastaser anbefaler behandlingsintensivering sammenlignet med den tidligere SWENOTECA IV-protokollen. Vi velger derfor å behandle EGCT med dårlig prognose som testikkelkreft med ikke-pulmonale viscerale metastaser med dårlig prognose.
Kirurgi
Hos pasienter med non-seminomatøs EGCT oppstått fra mediastinum er kirurgisk reseksjon av alle restmasser etter kjemoterapi obligatorisk og bør utføres uten forsinkelse etter kjemoterapi.
Den gjenværende mediastinale svulsten er ofte stor, og det finnes ofte andre restmasser. Histologiske analyser av kirurgisk fjernede restmasser hos disse pasientene har avdekket annen germinalcellekreft enn teratom hos 66 % av pasientene og teratom hos 22 % (Vuky et al., 2001). Oftere enn ved andre germinalcellesvulster finnes det teratom med malign transformasjon i restmasser. Dette er en entitet med spesielt dårlig prognose, og hvor kirurgi er den eneste sjansen for helbredelse. Hvis fullstendig reseksjon er teknisk mulig, synes kirurgi å være gunstig selv hos pasienter med forhøyede tumormarkører.
Hos pasienter med non-seminomatøs EGCT som oppstår fra retroperitoneum, har reseksjon av restmasser vist annen germinalcellekreft enn teratom hos 25 % av pasientene og teratom hos 16 % av pasientene. Dette er en høyere andel enn hos pasienter med restmasser etter behandling av gonadal metastatisk sykdom. En fullstendig bilateral RPLND er derfor berettiget hos disse pasientene.
Det er ikke sikkert at seminomatøs EGCT krever kirurgi etter kjemoterapi (Albany & Einhorn, 2013; Albany, Kesler, & Cary, 2019; Busch, Seidel, & Zengerling, 2016).
Indikasjonene for adjuvant kjemoterapi ved andre typer germinalcellekreft enn teratom ved restlesjoner hos pasienter i intermediær og dårlig prognosegruppe er usikre, da det ikke finnes sterke evidensbaserte data. Både adjuvant kjemoterapi umiddelbart etter kirurgi og tett overvåkning kan være alternativer. Det viktigste fokuset bør imidlertid være å oppnå radikal kirurgi av alle restlesjoner.
«Salvage»-behandling
«Salvage»-behandling av retroperitoneal EGCT følger anbefalingene for gonadal germinalcellekreft. For mediastinale non-seminomatøse tumorer fører «salvage»-kjemoterapi til langsiktig remisjon hos mindre enn 10 % av pasientene, og derfor bør man vurdere kirurgisk reseksjon selv om tumormarkørene er forhøyet (Albany & Einhorn, 2013; Bokemeyer et al., 2002; Busch et al., 2016).
Registrering og oppfølging
Det er et eget registreringsskjema for pasienter med ekstragonadal germinalcellekreft, men de andre skjemaene er de samme for primær testikulær og primær ekstragonadal lokalisasjon av svulsten. Oppfølgingsplanen er den samme som for metastatisk germinalcellekreft. Ved mediastinal EGCT bør bildediagnostikk med MR eller CT av mediastinum planlegges samtidig som bildediagnostikk av retroperitoneum.
Oppfølging
Sist faglig oppdatert: 15.04.2021
Klinisk undersøkelse er individuelt utformet.
Det primære målet med oppfølgingen er å stille diagnose ved residiv tidligst mulig, for å kunne kurere pasienten ved hjelp av så lite terapi som mulig. Et annet viktig mål er å diagnostisere komplikasjoner og prøve å lindre symptomer fra sykdommen og behandlingen. En adekvat oppfølging er avhengig av inngående kunnskap om sykdommen med hensyn til histologi, behandling og forventet tilbakefallsmodus samt mulige bivirkninger. Intervallene og modusen for kontroller blir vanligvis tilpasset stadium, histologi, behandling, typisk tidspunkt for risiko for tilbakefall og forventet risiko for bivirkninger.
Fra SWENOTECA-erfaringer er risikoen for residiv svært lav 5 år etter behandlingsslutt, med noen unntak angitt nedenfor. Denne protokollen forsvarer derfor kortere oppfølging enn tidligere SWENOTECA-protokoller. Adjuvant karboplatin ved seminom i klinisk fase I kan utsette metastatisk sykdom, og dermed bør disse pasientene ha ekstra oppfølgingskontroller etter 7 og 10 år. Følgende pasienter behandlet for metastatisk non-seminom bør ha ekstra oppfølgingskontroller etter 7 og 10 år: intermediær og dårlig prognose, vedvarende resttumor ved 5-års oppfølging, teratom i gjenværende tumorreseksjon, eller teratom i testikkelen og ingen RPLND. For noen pasienter med multiple residiv med teratom, og for de med gjenværende tumorer som ikke er tilgjengelige for reseksjon (non-seminom), kan det indikeres livslang oppfølging.
I tillegget presenteres tidsplanene for minimum oppfølging i henhold til en rekke standardscenarier, men man må huske på at en rekke pasienter må få individualiserte tidsplaner på grunn av situasjoner som ikke tas i betraktning i standardplanen.
Behandling av residiv eller progresjon etter standard kombinasjons-kjemoterapi
Sist faglig oppdatert: 15.04.2021
Det bør om mulig utføres histologisk verifisering, det er viktigst hvis tumormarkørene er negative.
Behandlingen avhenger av residivlokalisasjon og gjennomgått behandling.
Oppfølgingsskjemaet i Swenoteca X må fylles ut og sendes til det regionale testikkelkreftregisteret umiddelbart hvis det oppdages et tilbakefall.
Det er svært viktig å oppdage eventuelle avvik fra postulerte residivrater og -mønstre så tidlig som mulig, for å kunne justere behandlings- og/eller oppfølgingsprogrammet, hvis det er indikert.
Residiv etter overvåkning eller adjuvant kjemoterapi
Sist faglig oppdatert: 15.04.2021
Residiv etter initialt klinisk stadium I bør behandles som initial metastatisk sykdom, i henhold til stadium og prognostisk gruppe. Se Kjemoterapi. Et mistenkt markørnegativt residiv bør bekreftes før oppstart av systemisk behandling.
Residiv etter initial strålebehandling
Sist faglig oppdatert: 15.04.2021
Residiv etter initialt klinisk stadium I eller stadium II behandlet med strålebehandling, bør behandles som initial metastatisk sykdom med kjemoterapi eller kirurgi, i henhold til klinisk stadium og prognostisk gruppe. Se Kjemoterapi.
Prognose for residiv eller progresjon
Sist faglig oppdatert: 15.04.2021
En publikasjon fra International Prognostic Factors Study Group, som brukte data fra 1984 pasienter med residiv, identifiserte prognostiske variabler hos pasienter med tilbakefall etter kjemoterapi med konvensjonell dosering (Lorch, Beyer, et al., 2010). Pasienter fra SWENOTECA-gruppen var inkludert i analysen. Disse variablene danner IPFSC-skåren som kan bidra til å klassifisere pasienter i prognostiske kategorier med hensyn til PFS og OS. Pasienter i kategorien svært lav risiko (bare seminom) eller prognosegrupper med lav risiko har en 2-års PFS > 50 %, og 3-års OS på >65 %. Prognosegruppene med intermediær, høy og svært høy risiko har 2-års PFS på henholdsvis 40 %, 26 % og 6 %, og 3-års OS på henholdsvis 58 %, 27 % og 6 %. Det har blitt publisert en retrospektiv studie, fra samme gruppe, som ser på utfallet av «salvage»-behandling hos 1594 pasienter (Lorch et al., 2011). Analysen indikerte at høydose karboplatinbasert «salvage»-behandling kan være nyttig for pasienter med tanke på både PFS og OS. Fordelen for OS ble sett i prognosegruppene med intermediær, høy og svært høy risiko.
|
Skåringspoeng |
|||||
|---|---|---|---|---|---|
| Parameter | -1 | 0 | 1 | 2 | 3 |
| Primærlokalisasjon | Gonadal | Ekstragonadal | Mediastinal NSGCT |
||
| Tidligere respons | CR/PRm- | PRm+/SD | PD | ||
| PFI, måneder | > 3 | ≤ 3 | |||
| AFP-«salvage» | Normal | ≤ 1000 | > 1000 | ||
| hCG-«salvage» (ved residiv) |
≤ 1000 | > 1000 | |||
| LBB* | Nei | Ja | |||
| Primær histologi | Ren SGCT | Non-SGCT | |||
|
Omgruppering av skårsum i kategorier: * LBB = Lever-, skjelett- eller hjernemetastaser |
|||||
«Salvage»-kjemoterapi med konvensjonell dosering
Sist faglig oppdatert: 15.04.2021
«Salvage»-regimet som foretrekkes for tiden, er paklitakselbasert standarddose kjemoterapi (TIP). Hos pasienter med gunstige prognostiske egenskaper kan ca. 70 % av pasientene kureres av dette regimet (Kondagunta et al., 2005).
Flere andre regimer har kurerbart potensial ved residiv av germinalcellekreft. Disse inkluderer regimer som inneholder platina/etoposid/ifosfamid (McCaffrey et al., 1997), gemcitabin/oxaliplatin (De Giorgi et al., 2006; Kollmannsberger et al., 2004; Pectasides, Pectasides, Farmakis, Aravantinos, Nikolaou, Koumpou, Gaglia, Kostopoulou, Mylonakis, & Skarlos, 2004), gemcitabin/paklitaksel (Einhorn, Brames, Juliar, & Williams, 2007), gemcitabin/oxaliplatin/paklitaksel (Bokemeyer et al., 2008; De Giorgi et al., 2004), oxaliplatin/irinotekan (Pectasides, Pectasides, Farmakis, Aravantinos, Nikolaou, Koumpou, Gaglia, Kostopoulou, Mylonakis, Economopoulos, et al., 2004) og gemcitabin/cisplatin/paklitaksel (Nicolai et al., 2009).
EMA-CO kan være et alternativ hos pasienter med tilbakevendende sykdom som uttrykker ß-hCG, med noen pasienter som oppnår CR etter tilbakefall etter HDCT (Raggi et al., 2017).
«Salvage»-behandling med HDCT
Sist faglig oppdatert: 15.04.2021
Høydose kjemoterapi (HDCT) har i økende grad blitt brukt som «salvage»-behandling for pasienter med tilbakefall etter primær cisplatinbasert kjemoterapi. Flere fase I/II-studier og retrospektive studier har evaluert effekten av HDCT hos pasienter med tilbakefall og/eller cisplatin-refraktær sykdom. Det er betydelig variasjon i studiedesign, doseintensitet og pasientvalg, og dermed utfall: rapportert sviktfri overlevelse varierer fra 12 % til 63 %. Einhorn et al. har publisert den største retrospektive serien, som omfatter 184 pasienter behandlet med salvage tandem HDCT (karboplatin og etoposid) fra 1996 til 2004 (Einhorn, Williams, et al., 2007). Reseksjon av restmasser ble utført når det var teknisk mulig. Etter en median oppfølging på 48 måneder var 63 % kontinuerlig sykdomsfrie. Dette er en høyere andel enn tidligere rapportert i fase II-studier, og kan delvis forklares ved utelukkelse av pasienter med primære mediastinale svulster eller de med sene residiv. I tillegg forble 45 % av pasientene som var refraktære mot cisplatin, sykdomsfrie, noe som bekrefter at HDCT kan overvinne cisplatinresistens hos et betydelig antall pasienter.
Behandling av tilbakefall
Sist faglig oppdatert: 15.04.2021
Se flytskjema, Appendix XIII.
Alle pasienter med tilbakefall etter initial kjemoterapi for metastatisk sykdom bør umiddelbart henvises til et senter med erfaring i behandling av metastatiske germinalcellesvulster.
Hvis det oppdages et patologisk nivå av AFP, β-hCG eller PLAP uten tydelig metastase på CT thorax/abdomen/bekken, bør det utføres ytterligere MR-bildediagnostikk av hjerne og ryggrad. Det bør også gjennomføres en ultralydundersøkelse av den kontralaterale testikkelen.
Det bør utføres gjentatte tumormarkører for å utelukke falske positive funn.
Hvis det bekreftes sikre patologiske nivåer av de spesifikke tumormarkørene AFP, β-hCG (men ikke LDH) eller PLAP, med eller uten kliniske eller radiologiske tegn på metastaser, bør «salvage»-kjemoterapi innledes så snart som mulig.
Det er tilrådelig med biopsi av eventuelle åpenbare metastatiske/tumorlesjoner, men det er ikke obligatorisk hvis det er klar og vedvarende forhøyelse av tumormarkører i serum.
«Salvage»-behandling ved metastatisk sykdom
Sist faglig oppdatert: 15.04.2021
SWENOTECA bruker IPFSC-skåren, behandling med «salvage»-kjemoterapi bestemmes av prognosegruppen og gjennomgått behandling. Pasienter med gunstig prognoseskår vil mest sannsynlig bli kurert av et taxanbasert regime med konvensjonell dosering (TIP). I utvalgte tilfeller med nodulært seminomatøst tilbakefall med lite volum, kan imidlertid strålebehandling være et alternativ.
Pasienter med intermediær prognoseskår eller verre har en 2-års PFS på 40 %, og dermed anbefales høydose kjemoterapi som primær «salvage»-kjemoterapi.
Hvis det er indikasjoner på metastatisk sykdom ved bildediagnostikk uten forhøyet AFP, β-hCG eller PLAP i serum, bør det utføres biopsi/kirurgi for å oppnå histologisk verifisering. Hvis en mistenkt lesjon er relativt stabil og PET-negativ, kan lesjonen observeres nøye.
Det finnes mer informasjon i følgende underkapitler:
Kirurgi etter strålebehandling eller kjemoterapi
Sist faglig oppdatert: 15.04.2021
Non-seminom
I en tilbakefallssituasjon er kirurgisk fjerning av lesjoner etter «salvage»-kjemoterapi obligatorisk. For pasienter med tilbakefall etter initial metastatisk sykdom, er kirurgisk fjerning av gjenværende lesjoner obligatorisk hos pasienter med non-seminom.
Seminom
Konsoliderende behandling etter kjemoterapi i form av kirurgi eller strålebehandling bør ikke brukes med mindre det er verifisert ved biopsi at gjenværende lesjoner inneholder annen germinalcellekreft enn teratom. Selv PET-positive lesjoner bør underkastes biopsi siden PET kan være falsk positiv. Hos pasienter med avansert seminom kan kirurgi etter kjemoterapi være teknisk utfordrende, men om mulig bør det velges kirurgi fremfor konsoliderende strålebehandling.
Palliativ behandling
Sist faglig oppdatert: 15.04.2021
Det dør rundt ti pasienter av testikkelkreft i Norge og Sverige hvert år. Før en pasient klassifiseres som palliativ, bør pasienten diskuteres i SWENOTECA-nettverket. Responsen på palliativ behandling er ofte kort, og dermed er det ønske om symptomlindring (C. Oing et al., 2018). Strålebehandling vil nesten alltid gi god palliasjon av lokale symptomer.
Den kliniske enkeltarm fase II-studien «Cabazitaxel as salvage treatment for cisplatin-resistant germ cell cancer» undersøker effekten av cabazitaxel (EudraCT-nummer: 2012-004418-32). Et annet alternativ er oral etoposid 50 mg/m2 daglig, inntil progresjon eller uakseptabel toksisitet (Miller & Einhorn, 1990).
Palbociklib (eller andre CK4/6-hemmere) er sannsynligvis effektivt ved inoperabelt, voksende teratom, med median PFS ved 23 uker for pasienter med inoperabelt teratom i en liten fase II-studie (Vaughn et al., 2015). Så langt er erfaringen innen SWENOTECA med palbociclib i denne situasjonen svært begrenset, og kandidater bør diskuteres i SWENOTECA-nettverket.
For tiden har immunterapi ingen plass i behandlingen av refraktær sykdom (N. Adra et al., 2018).
Tyrosinkinasehemmere kan også være et alternativ, og noen pasienter opplever god respons (Necchi et al., 2017).
Seneffekter og oppfølging etter behandling for testikkelkreft
Sist faglig oppdatert: 15.04.2021
Pasientene bør forsikres om at den langsiktige generelle helserelaterte livskvaliteten i de fleste tilfeller er den samme som hos menn som ikke har gjennomgått behandling for testikkelkreft (esmo.org). Enkelte bivirkninger av testikkelkreftbehandling kan imidlertid dukke opp flere år etter behandlingen.
Når oppfølging for tilbakefall pågår:
- Kontroll av testosteron, SHBG, LH og FSH år 1, 3, 5 og opptil 10 år i henhold til oppfølgingsplaner, regelmessig for pasienter på substitusjonsterapi med testosteron og ved forekomst av symptomer på hypogonadisme for andre
- Metabolsk screening (lipider, fastende glukose, HbA1c og blodtrykk) ved 1 år og siste oppfølging
- Ved siste onkologiske oppfølging bør alle pasienter få utdelt en pasientinformasjon som oppsummerer den gjennomgåtte behandlingen, de viktigste seneffektene og anbefalingene for videre oppfølging, Appendix XVII.
Kontroller hos fastlegen
Sist faglig oppdatert: 15.04.2021
Hos pasienter behandlet med kjemoterapi for metastatisk sykdom eller strålebehandling anbefaler vi pasienten å gjennomgå regelmessige undersøkelser hos fastlegen minimum hvert 5. år etter at den onkologiske oppfølgingen er ferdig, og oftere ved patologiske funn. Formålet med disse kontrollene er å forebygge, identifisere og muligens behandle komplikasjoner etter den gjennomgåtte kreftbehandlingen. Disse kontrollene kan omfatte:
- Anamnese og initiering av primær eller sekundær profylakse angående kardiovaskulær risikoprofil (følger anbefalingene som gjelder for den generelle befolkningen), og symptomer på hypogonadisme, kardiovaskulær sykdom og fatigue
- Råd om livsstilsfaktorer som røykeslutt, sunt kosthold og fysisk aktivitet
- Måling av blodtrykk, høyde/vekt (BMI)
- Blodprøver: Fastende lipidprofil (totalkolesterol, HDL- og LDL-kolesterol, triglyserider), glukose, HbA1c og kjønnshormoner ved hypogonadale symptomer (testosteron, SHBG, LH og FSH)
Kardiovaskulær sykdom (CVD)
Sist faglig oppdatert: 15.04.2021
Kardiovaskulær dødelighet er høyere blant testikkelkreftoverlevere (TCS) enn i den generelle befolkningen (Fossa et al., 2007; Fossa, Aass, Harvei, & Tretli, 2004; Kvammen et al., 2016). Kardiovaskulære bivirkninger kan både være relatert til behandlingstoksisitet, og lavt testosteronnivå med økt risiko for metabolsk syndrom (Haugnes et al., 2010; van den Belt-Dusebout et al., 2006). Menn som tidligere har blitt behandlet med cisplatinbasert kjemoterapi, har 2–3 ganger økt risiko for kardiovaskulær sykdom sammenlignet med menn behandlet med kirurgi eller den generelle befolkningen i flere studier. Risikoen for kardiovaskulær sykdom er også økt etter infradiafragmatisk stråling, men resultatene er motstridende (Haugnes et al., 2010; Huddart et al., 2003; van den Belt-Dusebout et al., 2006). Den absolutte risikoen for kardiovaskulær sykdom > 10 år etter cytotoksisk behandling er 6–10 % (Haugnes et al., 2010; Huddart et al., 2003). Kombinasjon av både kjemoterapi og strålebehandling er spesielt skadelig, med en absolutt risiko for kardiovaskulær sykdom på 20 % > 10 år etter behandling (Haugnes et al., 2010). Cisplatinbasert kjemoterapi er også forbundet med økt forekomst av hypertensjon og metabolsk syndrom, mens strålebehandling er forbundet med økt forekomst av diabetes (Haugnes et al., 2010; Haugnes et al., 2007; Nuver et al., 2005).
Endoteliale og inflammatoriske markører som fibrinogen og von Willebrand-faktor er økt hos menn tidligere behandlet med cisplatin-basert cytostatika, mens høysensitivt C-reaktivt protein (hs-CRP) er forhøyet mange år etter gjennomgått strålebehandling (Haugnes et al., 2010; Nuver et al., 2004; Wethal et al., 2007). Disse funnene antyder at endotelial dysfunksjon kan være et mulig bindeledd mellom cytotoksisk behandling og aterosklerose.
Sekundær kreft
Sist faglig oppdatert: 15.04.2021
Det er fare for ny germinalcellekreft i den kontralaterale testikkelen, og 2–5 % av testikkelkreftpasienter utvikler en ny germinalcellekreft i gjenværende testikkel (Andreassen, Grotmol, Cvancarova, Johannesen, & Fossa, 2011).
Det er økt risiko for sekundære non-germinalcelle maligne neoplasmer etter cytotoksisk behandling for testikkelkreft (Kvammen et al., 2019), med en betydelig latenstid fra kreftbehandling til forekomst av sekundær kreft (Hellesnes et al., 2019; Kvammen et al., 2016). Den økte risikoen for andre kreftformer etter cisplatinbasert kjemoterapi eller strålebehandling er rapportert å være 40–80 % (Fung, Fossa, Milano, Oldenburg, & Travis, 2013; Groot et al., 2018; Hellesnes et al., 2019; Kier et al., 2016), med enda høyere økt risiko hos pasienter som har fått kombinert kjemoterapi og strålebehandling (standardisert insidensrate > 2). Det er rapporter økt risiko for kreft i blære, nyre, lunge og bløtvev etter 2 sykluser eller mer med cisplatinbasert kjemoterapi. Adjuvant kjemoterapi med BEP x 1 og karboplatin x 1 har ikke vist seg å øke risikoen for andre kreftformer, men observasjonstiden er begrenset med en median oppfølging på 9,5 år (Hellesnes et al., 2019).
Strålebehandling har ført til økt risiko for kreft i organer lokalisert i eller i nærheten av tidligere strålefelt , inkludert mage-tarmkanal, bukspyttkjertel, lever, lunge, nyre og blære.
I en nyere stor norsk studie blant 5 600 testikkelkreftoverlevere ble det rapportert om 28 % økt risiko for sekundær kreft etter kirurgi alene sammenlignet med den generelle befolkningen, med signifikant økt risiko for skjoldbruskkjertelkreft og melanom. Den økte risikoen for sekundær kreft etter kirurgi alene kan delvis forklares av overvåkningsbias. En genetisk følsomhet og/eller miljøfaktorer som predisponerer for testikkelkreft samt andre maligniteter, vil imidlertid sannsynligvis være medvirkende faktorer (Del Risco Kollerud et al., 2018).
Fertilitet og hypogonadisme
Sist faglig oppdatert: 15.04.2021
Fertilitet
- Alle menn i alderen < 56 år som ønsker å bli fedre, bør tilbys kryopreservering av sæd siden subfertilitet er vanlig blant menn diagnostisert med testikkelkreft (Moller & Skakkebaek, 1999).
Cytotoksisk behandling kan påvirke både fertiliteten og nivåene av kjønnshormoner negativt (DeSantis, Albrecht, Holtl, & Pont, 1999). Resultater fra en stor norsk oppfølgingsstudie blant testikkelkreftoverlevere har vist at fertiliteten reduseres med økende behandlingsintensitet (Brydoy et al., 2005). Likevel hadde nesten halvparten av mennene behandlet med store kumulative cisplatindoser blitt fedre etter testikkelkreftbehandling uten å bruke kryopreservert sæd. Behandling med karboplatin påvirket ikke kjønnshormoner eller spermatogenese negativt i en studie av 54 behandlede menn (Ghezzi et al., 2016). I tillegg fant en nyere svensk studie ingen langsiktige påvirkning på sædkvaliteten etter adjuvant kjemoterapi med BEP x 1 eller karboplatin x 1 (Weibring et al., 2019).
Unnfangelse vurderes som trygt seks måneder etter avsluttet kjemoterapi. Kjemoterapi og/eller strålebehandling økte ikke risikoen for misdannelser hos barn med en far som var behandlet for testikkelkreft, sammenlignet med menn som bare var under overvåkning (Al-Jebari et al., 2019).
Hvis en pasient unnfanger i løpet av eller kort tid (< 6 måneder) etter å ha gjennomgått kjemoterapi, bør den potensielle risikoen for fosteret diskuteres grundig med eksperter innen feltet. De kliniske dataene er for sparsomme og utilstrekkelige til å kunne gi en standard anbefaling om hvordan man håndterer en slik graviditet.
Retrograd ejakulasjon
Retrograd ejakulasjon forekommer hos 59 % av pasientene etter bilateral RPLND og 32 % etter unilateral RPLND (Gerdtsson et al., 2019). Pasientene bør informeres før kirurgi. Forekomsten har blitt redusert etter at nervesparende operasjonsteknikk ble introdusert (Jacobsen et al., 1999).
For menn som ønsker å bli fedre bør behandling med α-sympatomimetika som fenylpropanolamin eller imipramin vurderes ettersom disse stoffene kan reversere retrograd utløsning (Kamischke & Nieschlag, 2002).
Hypogonadisme
Det er viktig å identifisere symptomer knyttet til hypogonadisme og tilby behandling for å lindre symptomene. Primær endokrin hypogonadisme er utbredt hos 5–13 % av pasientene etter orkiektomi, og øker til 11–27 % etter kjemoterapi (Nord et al., 2003) (ESMO).
Typiske symptomer:
- Redusert libido
- Ereksjonssvikt
- Gynekomasti
- Redusert behov for barbering
- Energiløshet
- Dystymi
- Redusert muskelstyrke
- Økt kroppsmasseindeks
Endokrin hypogonadisme er assosiert med hypertensjon, fedme, metabolsk syndrom og diabetes (Laaksonen et al., 2004; Svartberg et al., 2004) og antagelig også med økt dødelighet (Laughlin, Barrett-Connor, & Bergstrom, 2008; Shores, Matsumoto, Sloan, & Kivlahan, 2006).
Undersøkelser
Det bør utføres to målinger av kjønnshormoner om morgenen for å bekrefte hypogonadisme (Gupta, Lindemulder, & Sathyan, 2000). SHBG, LH og FSH bør evalueres i tillegg til testosteron. Fri testosteronindeks kan beregnes (testosteron x 10/SHBG), siden dette kan gjenspeile det biologisk aktive testosteronnivået bedre.
Behandling
- Den nåværende anbefalingen er at testikkelkreftoverlevere med gjentatte lave testosteronnivåer (i henhold til lokale laboratorieretningslinjer og alder) (Bjerner, Biernat, Fossa, & Bjoro, 2009) OG kliniske symptomer bør tilbys behandling med testosteronsubstitusjon i en prøveperiode på tre til seks måneder (ESMO) og deretter vurdere effekten. Testosteronsubstitusjon administreres for å lindre hypogonadale symptomer, og det finnes ingen data som indikerer redusert kardiovaskulær risiko med substitusjon.
- Etter bilateral orkiektomi og ved etablert testosteronmangel etter behandling for GCNIS, er livslang testosteronsubstitusjon nødvendig.
- Menn med betydelige kliniske symptomer (redusert libido, erektil dysfunksjon, energiløshet), men med testosteronnivå innenfor normalområdet, kan ha nytte av testosteronsubstitusjon. Ved hypogonadale symptomer og betydelig forhøyet LH men normalt testosteronnivå, bør kompensert hypogonadisme og potensiell behandling vurderes. Testosteronsubstitusjon i disse to situasjonene bør diskuteres med en endokrinolog.
Behandling med injeksjoner hver 10.–12. uke eller daglig transdermal gel er aktuelle alterntiver og individuelle vurderinger bør styre beslutninger, dose og behandlingsintervall. Prostatakreft bør utelukkes før behandling (PSA) og hematokrit bør være under 53 %. Kongestiv hjertesvikt bør være godt medisinert. For menn som ønsker å bli fedre, vil substitusjon med testosteron redusere sædens levedyktighet og det bør vurderes forsinket oppstart av behandlingen. En endokrinolog eller androlog kan konsulteres. Hematokrit og blodtrykk bør vurderes under oppfølgingen.
Fatigue
Sist faglig oppdatert: 15.04.2021
Fatiguerelaterte symptomer er de mest plagsomme symptomene blant langtids testikkelkreftoverlevere, og 49 % av overleverne rapporterte energiløshet (Oechsle et al., 2016). Kronisk fatigue er definert som en plagsom, vedvarende subjektiv følelse av fysisk, emosjonell og/eller mental tretthet relatert til kreft og/eller kreftbehandling (Bower et al., 2014). Merk at det ikke er mulig å måle kronisk fatigue objektivt, diagnosen bygger på egenrapportering, og flere instrumenter er tilgjengelige, f.eks. skjemaet Fatigue Questionnaire (FQ).
Forekomsten av kronisk fatigue har blitt evaluert i en langtidsstudie av 812 norske testikkelkreftoverlevere (Sprauten et al., 2015). Forekomsten av kronisk fatigue økte fra 15 % median 11 år etter behandling, til 27 % median 19 år etter behandling. Utbredt nevropati, Raynaud-lignende fenomener, lavt testosteron og høyere nivåer av angst og depresjon økt risikoen for kronisk fatigue, mens fysisk aktivitet hadde en beskyttende effekt mot kronisk fatigue. Merk at behandlingsmodalitet ikke var assosiert med kronisk fatigue i multivariate analyser.
Det anbefales at alle testikkelkreftoverlevere evalueres for symptomer på fatigue. For de som presenter seg med moderat til alvorlig fatigue, bør det utføres en mer omfattende vurdering, inkludert evaluering av, og muligens, behandling av medvirkende faktorer (f.eks. smerte, anemi, hypogonadisme) (Bower et al., 2014). Når det gjelder behandlingsstrategier, har fysisk aktivitet vist seg å redusere forekomsten av langvarig kronisk fatigue hos testikkelkreftoverlevere, og anbefales sterkt (Adams et al., 2018). Det finnes data som støtter bruk av psykososiale intervensjoner. Det finnes ingen data som støtter bruk av farmakologiske intervensjoner i behandlingen av kronisk fatigue, selv om randomiserte studier har vist effekt av open-label placebo for å forbedre kreftrelatert fatigue (Hoenemeyer, Kaptchuk, Mehta, & Fontaine, 2018; Zhou et al., 2019).
Andre seneffekter
Sist faglig oppdatert: 15.04.2021
Ototoksisitet og nevrotoksisitet kan oppstå etter cisplatinbehandling.
Et betydelig antall testikkelkreftoverlevere lider av andre langsiktige komplikasjoner (nefrotoksisitet, nevrotoksisitet, ototoksisitet, lungetoksisitet og psykososiale problemer) (Brydoy et al., 2009; Fossa, Aass, Winderen, Bormer, & Olsen, 2002; Haugnes et al., 2009). Både behandling med store cisplatindoser (>850 mg) og røyking øker risikoen for langsiktig ototoksisitet, nevrotoksisitet og lungetoksisitet (Widen & Erlandsson, 2004).
Ototoksisitet
Etter behandling av metastatisk sykdom med BEP rapporterer 20–25 % av pasientene langvarig nedsatt hørsel og tinnitus (Frisina et al., 2016), spesielt ved høyere frekvenser. Økende alder er en viktig faktor for hørselstap uavhengig av behandling (Haugnes et al., 2018). Faktorer som kan øke denne risikoen, inkluderer alvorlig støyeksponering før behandling, samtidig behandling med andre ototoksiske midler (som aminoglykosider) og unormal nyrefunksjon. Dessverre er det ennå ikke identifisert noen legemidler som lindrer symptomene (ESMO). Menn med behandlingsindusert ototoksisitet (tinnitus, nedsatt hørsel) bør unngå støyende miljøer
Det bør vurderes konsultasjon hos ørespesialist med tanke på tinnitusbehandling eller høreapparat.
Nevrotoksisitet
Perifer sensorisk nevropati ses hos 5 % av pasientene etter én syklus med BEP (Albers et al., 2008) og hos 25–35 % etter tre til fire sykluser med BEP (R. de Wit et al., 2001). Nevroprotektiv behandling har vært og blir for tiden testet, men ingen er inkludert i rutinemessig klinisk praksis (ESMO). Potensielle terapeutiske virkestoffer er duloksetin (Smith et al., 2013), trisykliske antidepressiva og antikonvulsiva (Jordan et al., 2020).
Raynauds fenomen
Raynauds fenomen kan forekomme hos pasienter som får bleomycin og/eller cisplatin og bør fortrinnsvis håndteres konservativt og ved å informere pasientene om dette. Ved alvorlige problemer kan det brukes lavdose kalsiumblokkere. Flere andre alternativer, for eksempel topikal nitroglyserin, kan også vurderes (Hinze & Wigley, 2018).
Kontroller hos fastlegen
Sist faglig oppdatert: 15.04.2021
Hos pasienter behandlet med kjemoterapi for metastatisk sykdom eller strålebehandling anbefaler vi pasienten å gjennomgå regelmessige undersøkelser hos fastlegen minimum hvert 5. år etter at den onkologiske oppfølgingen er ferdig, og oftere ved patologiske funn. Formålet med disse kontrollene er å forebygge, identifisere og muligens behandle komplikasjoner etter den gjennomgåtte kreftbehandlingen. Disse kontrollene kan omfatte:
- Anamnese og initiering av primær eller sekundær profylakse angående kardiovaskulær risikoprofil (følger anbefalingene som gjelder for den generelle befolkningen), og symptomer på hypogonadisme, kardiovaskulær sykdom og fatigue
- Råd om livsstilsfaktorer som røykeslutt, sunt kosthold og fysisk aktivitet
- Måling av blodtrykk, høyde/vekt (BMI)
- Blodprøver: Fastende lipidprofil (totalkolesterol, HDL- og LDL-kolesterol, triglyserider), glukose, HbA1c og kjønnshormoner ved hypogonadale symptomer (testosteron, SHBG, LH og FSH)
Kardiovaskulær sykdom (CVD)
Sist faglig oppdatert: 15.04.2021
Kardiovaskulær dødelighet er høyere blant testikkelkreftoverlevere (TCS) enn i den generelle befolkningen (Fossa et al., 2007; Fossa, Aass, Harvei, & Tretli, 2004; Kvammen et al., 2016). Kardiovaskulære bivirkninger kan både være relatert til behandlingstoksisitet, og lavt testosteronnivå med økt risiko for metabolsk syndrom (Haugnes et al., 2010; van den Belt-Dusebout et al., 2006). Menn som tidligere har blitt behandlet med cisplatinbasert kjemoterapi, har 2–3 ganger økt risiko for kardiovaskulær sykdom sammenlignet med menn behandlet med kirurgi eller den generelle befolkningen i flere studier. Risikoen for kardiovaskulær sykdom er også økt etter infradiafragmatisk stråling, men resultatene er motstridende (Haugnes et al., 2010; Huddart et al., 2003; van den Belt-Dusebout et al., 2006). Den absolutte risikoen for kardiovaskulær sykdom > 10 år etter cytotoksisk behandling er 6–10 % (Haugnes et al., 2010; Huddart et al., 2003). Kombinasjon av både kjemoterapi og strålebehandling er spesielt skadelig, med en absolutt risiko for kardiovaskulær sykdom på 20 % > 10 år etter behandling (Haugnes et al., 2010). Cisplatinbasert kjemoterapi er også forbundet med økt forekomst av hypertensjon og metabolsk syndrom, mens strålebehandling er forbundet med økt forekomst av diabetes (Haugnes et al., 2010; Haugnes et al., 2007; Nuver et al., 2005).
Endoteliale og inflammatoriske markører som fibrinogen og von Willebrand-faktor er økt hos menn tidligere behandlet med cisplatin-basert cytostatika, mens høysensitivt C-reaktivt protein (hs-CRP) er forhøyet mange år etter gjennomgått strålebehandling (Haugnes et al., 2010; Nuver et al., 2004; Wethal et al., 2007). Disse funnene antyder at endotelial dysfunksjon kan være et mulig bindeledd mellom cytotoksisk behandling og aterosklerose.
Sekundær kreft
Sist faglig oppdatert: 15.04.2021
Det er fare for ny germinalcellekreft i den kontralaterale testikkelen, og 2–5 % av testikkelkreftpasienter utvikler en ny germinalcellekreft i gjenværende testikkel (Andreassen, Grotmol, Cvancarova, Johannesen, & Fossa, 2011).
Det er økt risiko for sekundære non-germinalcelle maligne neoplasmer etter cytotoksisk behandling for testikkelkreft (Kvammen et al., 2019), med en betydelig latenstid fra kreftbehandling til forekomst av sekundær kreft (Hellesnes et al., 2019; Kvammen et al., 2016). Den økte risikoen for andre kreftformer etter cisplatinbasert kjemoterapi eller strålebehandling er rapportert å være 40–80 % (Fung, Fossa, Milano, Oldenburg, & Travis, 2013; Groot et al., 2018; Hellesnes et al., 2019; Kier et al., 2016), med enda høyere økt risiko hos pasienter som har fått kombinert kjemoterapi og strålebehandling (standardisert insidensrate > 2). Det er rapporter økt risiko for kreft i blære, nyre, lunge og bløtvev etter 2 sykluser eller mer med cisplatinbasert kjemoterapi. Adjuvant kjemoterapi med BEP x 1 og karboplatin x 1 har ikke vist seg å øke risikoen for andre kreftformer, men observasjonstiden er begrenset med en median oppfølging på 9,5 år (Hellesnes et al., 2019).
Strålebehandling har ført til økt risiko for kreft i organer lokalisert i eller i nærheten av tidligere strålefelt , inkludert mage-tarmkanal, bukspyttkjertel, lever, lunge, nyre og blære.
I en nyere stor norsk studie blant 5 600 testikkelkreftoverlevere ble det rapportert om 28 % økt risiko for sekundær kreft etter kirurgi alene sammenlignet med den generelle befolkningen, med signifikant økt risiko for skjoldbruskkjertelkreft og melanom. Den økte risikoen for sekundær kreft etter kirurgi alene kan delvis forklares av overvåkningsbias. En genetisk følsomhet og/eller miljøfaktorer som predisponerer for testikkelkreft samt andre maligniteter, vil imidlertid sannsynligvis være medvirkende faktorer (Del Risco Kollerud et al., 2018).
Fertilitet og hypogonadisme
Sist faglig oppdatert: 15.04.2021
Fertilitet
- Alle menn i alderen < 56 år som ønsker å bli fedre, bør tilbys kryopreservering av sæd siden subfertilitet er vanlig blant menn diagnostisert med testikkelkreft (Moller & Skakkebaek, 1999).
Cytotoksisk behandling kan påvirke både fertiliteten og nivåene av kjønnshormoner negativt (DeSantis, Albrecht, Holtl, & Pont, 1999). Resultater fra en stor norsk oppfølgingsstudie blant testikkelkreftoverlevere har vist at fertiliteten reduseres med økende behandlingsintensitet (Brydoy et al., 2005). Likevel hadde nesten halvparten av mennene behandlet med store kumulative cisplatindoser blitt fedre etter testikkelkreftbehandling uten å bruke kryopreservert sæd. Behandling med karboplatin påvirket ikke kjønnshormoner eller spermatogenese negativt i en studie av 54 behandlede menn (Ghezzi et al., 2016). I tillegg fant en nyere svensk studie ingen langsiktige påvirkning på sædkvaliteten etter adjuvant kjemoterapi med BEP x 1 eller karboplatin x 1 (Weibring et al., 2019).
Unnfangelse vurderes som trygt seks måneder etter avsluttet kjemoterapi. Kjemoterapi og/eller strålebehandling økte ikke risikoen for misdannelser hos barn med en far som var behandlet for testikkelkreft, sammenlignet med menn som bare var under overvåkning (Al-Jebari et al., 2019).
Hvis en pasient unnfanger i løpet av eller kort tid (< 6 måneder) etter å ha gjennomgått kjemoterapi, bør den potensielle risikoen for fosteret diskuteres grundig med eksperter innen feltet. De kliniske dataene er for sparsomme og utilstrekkelige til å kunne gi en standard anbefaling om hvordan man håndterer en slik graviditet.
Retrograd ejakulasjon
Retrograd ejakulasjon forekommer hos 59 % av pasientene etter bilateral RPLND og 32 % etter unilateral RPLND (Gerdtsson et al., 2019). Pasientene bør informeres før kirurgi. Forekomsten har blitt redusert etter at nervesparende operasjonsteknikk ble introdusert (Jacobsen et al., 1999).
For menn som ønsker å bli fedre bør behandling med α-sympatomimetika som fenylpropanolamin eller imipramin vurderes ettersom disse stoffene kan reversere retrograd utløsning (Kamischke & Nieschlag, 2002).
Hypogonadisme
Det er viktig å identifisere symptomer knyttet til hypogonadisme og tilby behandling for å lindre symptomene. Primær endokrin hypogonadisme er utbredt hos 5–13 % av pasientene etter orkiektomi, og øker til 11–27 % etter kjemoterapi (Nord et al., 2003) (ESMO).
Typiske symptomer:
- Redusert libido
- Ereksjonssvikt
- Gynekomasti
- Redusert behov for barbering
- Energiløshet
- Dystymi
- Redusert muskelstyrke
- Økt kroppsmasseindeks
Endokrin hypogonadisme er assosiert med hypertensjon, fedme, metabolsk syndrom og diabetes (Laaksonen et al., 2004; Svartberg et al., 2004) og antagelig også med økt dødelighet (Laughlin, Barrett-Connor, & Bergstrom, 2008; Shores, Matsumoto, Sloan, & Kivlahan, 2006).
Undersøkelser
Det bør utføres to målinger av kjønnshormoner om morgenen for å bekrefte hypogonadisme (Gupta, Lindemulder, & Sathyan, 2000). SHBG, LH og FSH bør evalueres i tillegg til testosteron. Fri testosteronindeks kan beregnes (testosteron x 10/SHBG), siden dette kan gjenspeile det biologisk aktive testosteronnivået bedre.
Behandling
- Den nåværende anbefalingen er at testikkelkreftoverlevere med gjentatte lave testosteronnivåer (i henhold til lokale laboratorieretningslinjer og alder) (Bjerner, Biernat, Fossa, & Bjoro, 2009) OG kliniske symptomer bør tilbys behandling med testosteronsubstitusjon i en prøveperiode på tre til seks måneder (ESMO) og deretter vurdere effekten. Testosteronsubstitusjon administreres for å lindre hypogonadale symptomer, og det finnes ingen data som indikerer redusert kardiovaskulær risiko med substitusjon.
- Etter bilateral orkiektomi og ved etablert testosteronmangel etter behandling for GCNIS, er livslang testosteronsubstitusjon nødvendig.
- Menn med betydelige kliniske symptomer (redusert libido, erektil dysfunksjon, energiløshet), men med testosteronnivå innenfor normalområdet, kan ha nytte av testosteronsubstitusjon. Ved hypogonadale symptomer og betydelig forhøyet LH men normalt testosteronnivå, bør kompensert hypogonadisme og potensiell behandling vurderes. Testosteronsubstitusjon i disse to situasjonene bør diskuteres med en endokrinolog.
Behandling med injeksjoner hver 10.–12. uke eller daglig transdermal gel er aktuelle alterntiver og individuelle vurderinger bør styre beslutninger, dose og behandlingsintervall. Prostatakreft bør utelukkes før behandling (PSA) og hematokrit bør være under 53 %. Kongestiv hjertesvikt bør være godt medisinert. For menn som ønsker å bli fedre, vil substitusjon med testosteron redusere sædens levedyktighet og det bør vurderes forsinket oppstart av behandlingen. En endokrinolog eller androlog kan konsulteres. Hematokrit og blodtrykk bør vurderes under oppfølgingen.
Fatigue
Sist faglig oppdatert: 15.04.2021
Fatiguerelaterte symptomer er de mest plagsomme symptomene blant langtids testikkelkreftoverlevere, og 49 % av overleverne rapporterte energiløshet (Oechsle et al., 2016). Kronisk fatigue er definert som en plagsom, vedvarende subjektiv følelse av fysisk, emosjonell og/eller mental tretthet relatert til kreft og/eller kreftbehandling (Bower et al., 2014). Merk at det ikke er mulig å måle kronisk fatigue objektivt, diagnosen bygger på egenrapportering, og flere instrumenter er tilgjengelige, f.eks. skjemaet Fatigue Questionnaire (FQ).
Forekomsten av kronisk fatigue har blitt evaluert i en langtidsstudie av 812 norske testikkelkreftoverlevere (Sprauten et al., 2015). Forekomsten av kronisk fatigue økte fra 15 % median 11 år etter behandling, til 27 % median 19 år etter behandling. Utbredt nevropati, Raynaud-lignende fenomener, lavt testosteron og høyere nivåer av angst og depresjon økt risikoen for kronisk fatigue, mens fysisk aktivitet hadde en beskyttende effekt mot kronisk fatigue. Merk at behandlingsmodalitet ikke var assosiert med kronisk fatigue i multivariate analyser.
Det anbefales at alle testikkelkreftoverlevere evalueres for symptomer på fatigue. For de som presenter seg med moderat til alvorlig fatigue, bør det utføres en mer omfattende vurdering, inkludert evaluering av, og muligens, behandling av medvirkende faktorer (f.eks. smerte, anemi, hypogonadisme) (Bower et al., 2014). Når det gjelder behandlingsstrategier, har fysisk aktivitet vist seg å redusere forekomsten av langvarig kronisk fatigue hos testikkelkreftoverlevere, og anbefales sterkt (Adams et al., 2018). Det finnes data som støtter bruk av psykososiale intervensjoner. Det finnes ingen data som støtter bruk av farmakologiske intervensjoner i behandlingen av kronisk fatigue, selv om randomiserte studier har vist effekt av open-label placebo for å forbedre kreftrelatert fatigue (Hoenemeyer, Kaptchuk, Mehta, & Fontaine, 2018; Zhou et al., 2019).
Andre seneffekter
Sist faglig oppdatert: 15.04.2021
Ototoksisitet og nevrotoksisitet kan oppstå etter cisplatinbehandling.
Et betydelig antall testikkelkreftoverlevere lider av andre langsiktige komplikasjoner (nefrotoksisitet, nevrotoksisitet, ototoksisitet, lungetoksisitet og psykososiale problemer) (Brydoy et al., 2009; Fossa, Aass, Winderen, Bormer, & Olsen, 2002; Haugnes et al., 2009). Både behandling med store cisplatindoser (>850 mg) og røyking øker risikoen for langsiktig ototoksisitet, nevrotoksisitet og lungetoksisitet (Widen & Erlandsson, 2004).
Ototoksisitet
Etter behandling av metastatisk sykdom med BEP rapporterer 20–25 % av pasientene langvarig nedsatt hørsel og tinnitus (Frisina et al., 2016), spesielt ved høyere frekvenser. Økende alder er en viktig faktor for hørselstap uavhengig av behandling (Haugnes et al., 2018). Faktorer som kan øke denne risikoen, inkluderer alvorlig støyeksponering før behandling, samtidig behandling med andre ototoksiske midler (som aminoglykosider) og unormal nyrefunksjon. Dessverre er det ennå ikke identifisert noen legemidler som lindrer symptomene (ESMO). Menn med behandlingsindusert ototoksisitet (tinnitus, nedsatt hørsel) bør unngå støyende miljøer
Det bør vurderes konsultasjon hos ørespesialist med tanke på tinnitusbehandling eller høreapparat.
Nevrotoksisitet
Perifer sensorisk nevropati ses hos 5 % av pasientene etter én syklus med BEP (Albers et al., 2008) og hos 25–35 % etter tre til fire sykluser med BEP (R. de Wit et al., 2001). Nevroprotektiv behandling har vært og blir for tiden testet, men ingen er inkludert i rutinemessig klinisk praksis (ESMO). Potensielle terapeutiske virkestoffer er duloksetin (Smith et al., 2013), trisykliske antidepressiva og antikonvulsiva (Jordan et al., 2020).
Raynauds fenomen
Raynauds fenomen kan forekomme hos pasienter som får bleomycin og/eller cisplatin og bør fortrinnsvis håndteres konservativt og ved å informere pasientene om dette. Ved alvorlige problemer kan det brukes lavdose kalsiumblokkere. Flere andre alternativer, for eksempel topikal nitroglyserin, kan også vurderes (Hinze & Wigley, 2018).
Stromale testistumorer
Sist faglig oppdatert: 15.04.2021
Stromale svulster er ikke inkludert i SWENOTECA-registeret.
Stromale testistumorer er sjeldne og utgjør bare 2–4 % av alle testikkelsvulster hos voksne. Dette er hovedsakelig svulster klassifisert som Leydigcelle-tumor og Sertolicelle-tumor. Det har imidlertid blitt klassifisert en rekke ulike svulster (Moch, Cubilla, Humphrey, Reuter, & Ulbright, 2016).
- Leydigcelle-tumor
- Malign Leydigcelle-tumor
- Sertolicelle-tumor
- Malign Sertolicelle-tumor
- Granulosacelle-tumor
- Thecom/fibrom
- Gonadoblastom (tumor med germinalceller og kjønnsstreng/gonadestroma tumores)
- Andre kjønnsstreng/gonadestroma tumorer
Siden dette er sjeldne tumorer, finnes det begrensede data, og følgende anbefalinger er hovedsakelig basert på retningslinjer fra EAU.
Leydigcelle-tumor
Sist faglig oppdatert: 15.04.2021
Leydigceller produserer testosteron regulert av LH (luteiniserende hormon). Leydigcelle-tumores utgjør ca. 1–3 % av alle testikkelsvulster (Ruf et al., 2020). Denne svulsten ses hovedsakelig hos menn mellom 30 og 60 år. Hos barn er de assosiert med Klinefelters syndrom og kan være bilaterale. Leydigcelle-tumor er den vanligste av alle stromale testistumorer. De er vanligvis benigne, men kan ha et malignt potensial. Maligne Leydigcelle-tumores kan karakteriseres av følgende histopatologiske egenskaper som anses som risikofaktorer (Fankhauser et al., 2019):
- Diameter > 5 cm
- Cellulær atypi
- Økt mitotisk rate
- Økt MIB-1-ekspresjon
- Nekrose
- Vaskulær invasjon
- Infiltrerende marginer
- DNA aneuploidi
Metastatisk sykdom vil alltid innebære malign sykdom og ca. 10 % av pasientene med Leydigcelle-tumor har metastatisk sykdom. Pasienter med Leydigcelle-tumores uten maligne egenskaper har svært lav risiko for metastaser.
Diagnose
Leydigcelle-tumor debuterer ofte som en forstørret testikkel. Hos hele 80 % foreligger det hormonelle forstyrrelser i form av lavt testosteron, økt østradiol og økte nivåer av LH og FSH (Mineur, De Cooman, Hustin, Verhoeven, & De Hertogh, 1987). Dette kan forklare hvorfor ca. 30 % av pasientene også har symptomer i form av gynekomasti. Leydigcelle-tumores må skilles fra multinodulære og ofte bilaterale lesjoner som følger med androgenitalt syndrom (Rutgers, Young, & Scully, 1988).
Diagnostisk utredning omfatter blodprøver med markører for testikkelkreft (AFP, ß-hCG, LDH) og hormoner (testosteron, LH, FSH, østradiol og progesteron), ultralydundersøkelse av begge testikler samt CT thorax/abdomen/bekken.
Behandling
Det utføres vanligvis standard orkiektomi. Organsparende kirurgi kan vurderes spesielt ved bilaterale svulster (Paffenholz, Pfister, & Heidenreich, 2020). Ved histopatologiske tegn på malignitet bør adjuvant RPLND vurderes for å hindre metastaserende sykdom (Di Tonno et al., 2009). I klinisk stadium 2 anbefales RPLND selv om den langsiktige prognosen kan være dårlig (Calaway et al., 2019). Pasienter med metastatisk sykdom responderer dårlig på både kjemoterapi og strålebehandling. Kirurgi må derfor overveies.
Sertolicelle-tumor
Sist faglig oppdatert: 15.04.2021
Sertolicelle-tumores utgjør mindre enn 1 % av testikkelsvulster. Gjennomsnittsalder på diagnosetidspunktet er omkring 45 år og forekommer bare sjeldent under 20 år. Sistnevnte er assosiert med androgen resistens syndrom og Peutz-Jeghers syndrom (Mooney & Kao, 2018). Sertolicellene er støtteceller i sædkanalene i testikkelen og gir næring til modnende sædceller. Omtrent 10–20 % av Sertolicelle-tumores er maligne, og 10–12 % presenterer seg med metastatisk sykdom (Grogg et al., 2020).
Følgende karakteristika regnes som tegn på malignitet:
- Diameter > 5 cm
- Nukleær atypi
- Økt mitotisk aktivitet
- Nekrose
- Vaskulær invasjon
Metastatisk sykdom er sjelden selv med de ovennevnte maligne kjennetegnene. Det er beskrevet tre undergrupper: Storcellet kalsifiserende Sertolicelle-tumor, initratubulær storcellet hyaliniserende Sertolicelle-tumor og malign Sertolicelle-tumor (Moch et al., 2016; Mooney & Kao, 2018).
Diagnose
De fleste Sertolicelle-tumores er unifokale. Hormonelle forstyrrelser forekommer sjelden, og tumormarkørene AFP og ß-hCG er alltid innenfor normalområdet. Den diagnostiske utredningen er som beskrevet ovenfor for Leydigcelle-tumores.
Behandling
Det anbefales orkiektomi eller organsparende kirurgi, sistnevnte spesielt ved små bilaterale svulster. Organsparende kirurgi er bare et alternativ hvis gjenværende testikkelvev har tilstrekkelig endokrin og eksokrin funksjon. Hvis svulsten har maligne kjennetegn, kan adjuvant RPLND vurderes. Pasienter med metastatisk sykdom responderer dårlig på både kjemoterapi og strålebehandling.
Granulosacelletumor
Sist faglig oppdatert: 15.04.2021
Granulosacellesvulster i testiklene hos voksne er sjeldne og forekommer med isolert unilateral testikkelmasse og av og til med gynekomasti (Dieckmann, Bertolini, & Wulfing, 2019).
Omtrent 10–15 % av granulosacellesvulster hos voksne er maligne og RPLND anbefales dersom det påvises metastaser til retroperitoneale lymfeknuter. Maligne svulster er generelt store (> 4 cm), med økt mitose, nekrose og lymfovaskulær invasjon.
Fibrothecom
Sist faglig oppdatert: 15.04.2021
Fibrothecom anses benigne og kureres med eksisjon (Zhang, Kao, Ulbright, & Epstein, 2013).
Gonadoblastom
Sist faglig oppdatert: 15.04.2021
Klassisk gonadoblastom forekommer nesten utelukkende i dysgeniske gonader hos personer med forstyrrelser i kjønnsutviklingen. 40 prosent av disse svulstene er bilaterale. En variant inneholder elementer av både kjønnsstreng og germinalceller, og prognosen bestemmes av germinalcellekomponenten (Roth & Cheng, 2018). I disse tilfellene anbefales behandling og oppfølging som for germinalcellekreft.
Andre testistumorer
Sist faglig oppdatert: 15.04.2021
Prepubertalt teratom har benign atferd og anses å være non-GCNIS-relatert (Mooney & Kao, 2018). Det forekommer hovedsakelig hos barn og er dermed ikke dekket av dette programmet. Prepubertalt teratom kan imidlertid også forekomme hos voksne og bør klassifiseres som en benign svulst.
Spermatocytiske svulster
Sist faglig oppdatert: 15.04.2021
Spermatocytiske svulster er sjeldne og gjennomsnittsalderen er 55 år. Duplikasjon av kromosom 9 er det eneste konsekvente kromosomavviket ved spermatocytiske svulster. Siden disse svulstene aldri metastaserer, er behandlingen orkiektomi alene, og det er ikke nødvendig med oppfølging (R. Hu, Ulbright, & Young, 2019; Sharmeen, Rosenthal, & Howard, 2014). Et unntak er svulster som har gjennomgått sarkomatøs transformasjon som kan metastasere, og kan kreve aggressiv multimodal behandling.
Oppfølging
Sist faglig oppdatert: 15.04.2021
Pasienter med svulster klassifisert som benigne, trenger ikke følges opp med tanke på risiko for metastatisk sykdom. Pasienter bør imidlertid informeres om at det kan oppstå fremtidig hypogonadisme.
Pasienter med svulster med maligne histologiske egenskaper bør overvåkes med abdominal bildediagnostikk hver sjette måned i tre år.
Strålebehandling
Sist faglig oppdatert: 15.04.2021
Foreskrivning, registrering og rapportering av dose bør gjøres i henhold til ICRU-rapport 50 og supplerende ICRU-rapporter 62 og 83 (ICRU, 1993, 2010; Measurements, 1999).
Felt og doser
Sist faglig oppdatert: 15.04.2021
Seminom klinisk stadium I
Anbefalt regime 1:
1,8 Gy x 14, til totalt 25,2 Gy, fordelt på 5 fraksjoner per uke, til paraaortale og ipsilaterale iliaca communis og iliaca externa lymfeknuter.
Alternativ regime 2:
2 Gy x 10, til totalt 20 Gy, fordelt på 5 fraksjoner per uke, til paraaortale lymfeknuter
Merk: Ved T4-tumor eller gjennomgått inguinal eller skrotal kirurgi, inkluderes også ipsilaterale inguinale lymfeknuter.
Seminom klinisk stadium II
Sist faglig oppdatert: 15.04.2021
Seminom klinisk stadium II A:
2 Gy x 15, til totalt 30 Gy, fordelt på 5 fraksjoner per uke, til paraaortale og ipsilaterale iliaca communis og iliaca externa lymfeknuter.
Klinisk stadium II B:
2 Gy x 15 som ved CS IIA. I tillegg skal patologiske lymfeknuter ha en tilleggsdose (boost) på 2 Gy x 3 til totalt 6 Gy. SIB skal helst brukes.
Merk: Ved T4-tumor eller gjennomgått inguinal eller skrotal kirurgi, inkluderes også ipsilaterale inguinale lymfeknuter.
Pasientposisjon og fiksering
Sist faglig oppdatert: 15.04.2021
Pasienten plasseres i ryggleie og fikseres i henhold til lokal praksis for å sikre reproduserbar posisjonering gjennom hele behandlingen. Marker orkiektomiarret med en blytråd. For pasienter i reproduktiv alder bør kontralaterale testis skjermes med bly mot ekstern spredt stråling. Penis skal flyttes ut av behandlingsfeltene.
Strålebehandlingsteknikk
Sist faglig oppdatert: 15.04.2021
CT-veiledet 3-dimensjonell (3D) doseplan er obligatorisk. Standard behandling har vært via to motgående anteriorposterior-postererioranterior, AP-PA, felt. Bruk av VMAT-teknikk (Volumetric Modulated Arc Therapy) reduserer avgitt dose til beinmarg, men øker gjennomsnittlig dose samt dose avgitt til 50 % av nyrer, dose til lever og tarm sammenlignet med to AP-PA felt (Zilli et al., 2011) (som kan øke risikoen for sekundær malignitet (Hall & Wuu, 2003; Simone et al., 2012). På grunn av svært god langtidsoverlevelse til pasienter med germinalcellesvulster må det utvises stor forsiktighet med tanke på dose avgitt til risikoorganer.
Den CT-baserte feltplanen baseres på karenes forløp fordi lymfeknutene følger kar som aorta, vena cava inferior, ipsilaterale nyrevene og ipsilaterale bekkenvener (iliaca communis og externa). En prospektiv kohortstudie utført av German Testicular Cancer Study Group viste ingen tilbakefall i bekken med en modifisert nedre iliacale feltgrense til acetabulums øvre begrensing, som også anbefales av EGCCCG (Bamberg et al., 1999; Classen et al., 2003). Kraniell feltgrense er øvre dekkflate av Th12 (Wilder, Buyyounouski, Efstathiou, & Beard, 2012).
Strålekvalitet
3D-CRT (tredimensjonal konvensjonell stråleterapi) skal leveres med minimum 10 MV fotoner.
Målvolumer og volum av risikoorganer (OAR)
GTV (Gross tumour volume) defineres som volumet av enhver forstørret metastatisk lymfeknute (dvs. klinisk stadium IIA).
Målvolumer
CTV av den paraaortale region skal omfatte paraaortale lymfeknuter fra øvre dekkflate av Th 12 til aortabifurkaturen og defineres som kombinert volum av vena cava inferior og aorta inklusive synlige lymfeknuter og ethvert GTV. Til det kombinerte volumet legges det til 1,4 cm margin. Tilsvarende tegnes det inn volum for ipsilaterale nyrevene uten lateral margin.
Hvis ipsilaterale iliaca communis og iliaca externa lymfeknuter skal behandles, utvides CTV til det kombinerte volumet av iliaca communis- og iliaca externa-karene ned til øvre begrensning av foramen obturatorium inklusive synlige lymfeknuter og ethvert GTV med 1,4 cm margin i alle retninger.
Ved tidligere inguinal eller skrotal kirurgi eller ved en sjelden T4 tumor skal CTV omfatte både ipsilaterale iliaca communis og iliaca externa og ipsilaterale inguinale lymfeknuter med tilleggsmarginer som beskrevet i tidligere avsnitt.
CTV bør justeres for å unngå skjelett, tarm, muskel og blære.
ITV (internal target volume) settes likt med CTV da organbevegelsen her er neglisjerbar.
PTV (planning target volume) defineres i henhold til ICRU-definisjonen.
Risikoorganer
Begge nyrer skal tegnes inn i hvert CT-bilde som risikoorganer. Ikke mer en 25 % av noen nyre skal ha mer enn 20 Gy stråledose.
For strålebehandling av hjernemetastaser, se Appendix XI og Appendix XII.
Pasientinformasjon
Sist faglig oppdatert: 15.04.2021
Skriftlig informasjon om behandlingsalternativer må gis til alle pasienter, med tilstrekkelig tid for spørsmål. I tillegg bør det gis både muntlig og skriftlig informasjon om registrering i SWENOTECA-databasen. Pasienter kan velge å ikke la seg registrere. Pasienten skal behandles og følges i henhold til de samme prinsippene uavhengig av om han samtykker til registrering eller ikke.
I Norge bes pasientene om å signere den skriftlige pasientinformasjonen, og pasientene blir registrert ved universitetssykehusene i Oslo, Bergen, Trondheim og Tromsø.
I Sverige gjøres dette i det svenske testikkelkreftregisteret, som er et offisielt nasjonalt kvalitetsregister, der SWENOTECA overvåker journalene.
Umiddelbart etter at det er gitt informert samtykke/ikke gitt samtykke, skal SWENOTECA «Registreringsblankett» fylles ut.
I Sverige: Hvert behandlingssykehus er ansvarlig for denne registreringen.
Prosess og metode for utarbeidelse av retningslinjene
Hva er nasjonale retningslinjer?
Sist faglig oppdatert: 15.04.2021
I henhold til Nasjonal helseplan (2007–2010) har Helsedirektoratet en normerende rolle for helsetjenesten på tvers av helseregioner og tjenestenivå. Helsedirektoratet har en koordinerende rolle for å utvikle overordnede referanserammer for kreftomsorgen, sammen med de regionale helseforetakene, kommunene og andre relevante myndighetsorganer og tjenester.
Lov om kommunale helse- og omsorgstjenester § 12-5 fastslår at Helsedirektoratet er eneste aktør med mandat til å utvikle, formidle og vedlikeholde nasjonale faglige retningslinjer og veiledere som understøtter de mål som er satt for helse- og omsorgstjenesten.
De nasjonale faglige retningslinjene inneholder systematisk utviklede faglige anbefalinger som etablerer en nasjonal standard for utredning, behandling og oppfølging av pasientgrupper, diagnosegrupper og brukergrupper. Nasjonale faglige retningslinjer er et virkemiddel for å bidra til at helse- og omsorgstjenestene:
- Har god kvalitet
- Gjør riktige prioriteringer
- Ikke har uønsket variasjon i tjenestetilbudet
- Løser samhandlingsutfordringer
- Tilbyr helhetlige pasientforløp
Nasjonale faglige retningslinjer gir uttrykk for hva som anses som god praksis på utgivelsestidspunktet. Sentrale fagmiljøer og tjenestemottakere er aktivt involvert i utarbeidelsen.
Helsepersonell og alle deler av helse- og omsorgstjenesten er forpliktet til å yte forsvarlig helsehjelp. Retningslinjer, anerkjent fagkunnskap og allmenngyldige samfunnsetiske normer inngår som aksepterte grunnlag for vurdering av hva som er faglig forsvarlig. Retningslinjer er ment som et hjelpemiddel ved avveiningene tjenesteyterne må gjøre for å oppnå forsvarlig og god kvalitet i tjenesten. De er ikke rettslig bindende, men faglig normerende for valg man anser fremmer kvalitet, god praksis og likhet i tjenesten på utgivelsestidspunktet. Helsepersonell må likevel vise faglig skjønn i vurderingen av hver enkelt pasient for å ta hensyn til individuelle behov. Dersom helsepersonell eller institusjoner velger å fravike anbefalinger i en retningslinje, skal dette dokumenteres og begrunnes.
Helsetjenestens eiere og ledelse har ansvar for tilrettelegging av virksomheten slik at anbefalinger gitt i nasjonale faglige retningslinjer kan følges.
Kunnskapsbasert prosess
Sist faglig oppdatert: 15.04.2021
Helsedirektoratet legger til grunn at alle nasjonale retningslinjer skal være utarbeidet etter en metode med vekt på forskningsbasert kunnskap, tydelig og tilgjengelig dokumentasjon, brukermedvirkning, tverrfaglighet, fokus på praksis, implementering og oppdatering. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av blære- og urotelkreft 103 Det er en omfattende prosess å lage gode retningslinjer som tilfredsstiller krav til prosess, metode og transparens som er det nivå Helsedirektoratet og andre internasjonale organisasjoner har lagt til grunn for utforming av anbefalinger. Helsedirektoratet ønsker i arbeidet med nasjonale retningslinjer for kreftbehandling å bygge på det arbeid faggruppene tilsluttet Onkologisk Forum i en årrekke har gjort med å lage behandlingsveiledere.
Gradering av kunnskapsgrunnlaget
Sist faglig oppdatert: 15.04.2021
Ved utarbeiding av nasjonale retningslinjer stilles det krav om at all relevant kunnskap på området hentes frem, beskrives og vurderes på en systematisk og åpen måte.
I de tre første versjonene av dette handlingsprogrammet ble gradering av evidensnivå (A–D) anvendt for å vise hvilket vitenskapelig grunnlag kunnskapen er basert på.
I disse retningslinjene er kun kunnskapsgrunnlaget og ikke anbefalingene gradert. For å indikere kunnskapsgrunnlaget for anbefalingene, er det brukt gradering A–D. En anbefaling som er anført med D behøver derfor ikke å være en svakere anbefaling enn en som er anført A, B eller C. Det henspeiler kun til kunnskapsgrunnlaget anbefalingen er basert på. I enkelte sammenhenger vil klinisk erfaring og gjeldende praksis være et godt grunnlag for anbefalingen. I andre sammenhenger er det uttrykk for at det ikke finnes tilstrekkelig vitenskapelig dokumentasjon for anbefalingen, selv om det hadde vært ønskelig.
| Studietype | Evidensnivå | Gradering av evidensnivå |
|---|---|---|
|
Kunnskap som bygger på systematiske oversikter og meta-analyser av randomiserte kontrollerte studier. Kunnskap som bygger på minst én randomisert kontrollert studie. |
Nivå 1a
Nivå 1b |
A |
|
Kunnskap som bygger på minst én godt utformet kontrollert studie uten randomisering. Kunnskap som bygger på minst én annen godt utformet kvasi-eksperimentell studie uten randomisering. |
Nivå 2a
Nivå 2b |
B |
| Kunnskap som bygger på godt utformede ikke eksperimentelle beskrivende studier, som sammenlignende studier, korrelasjonsstudier og case studier | Nivå 3 | C |
| Kunnskap som bygger på rapporter eller oppfatninger fra eksperter, komiteer og/eller klinisk ekspertise hos respekterte autoriteter | Nivå 4 | D |
I versjon 4 av handlingsprogrammet er kunnskapsgrunnlaget i liten grad gradert i henhold til tabellen over. Manglende gradering betyr ikke at kunnskapsgrunnlaget er manglende eller har mindre evidensnivå. Uavhengig av om gradering er angitt er er grunnlaget for anbefalingene gjennomgått med de samme krav til evidensnivå for en gitt anbefaling.
Bakgrunn og arbeidsprosess
Sist faglig oppdatert: 15.04.2021
Utvikling av nasjonale handlingsprogrammene for kreftbehandling er et viktig tiltak under Nasjonal strategi for kreftområdet (2006–2009) (forlenget til 2011. Og videre utvikling av de nasjonale handlingsoppgavene er en kjerneoppgave i oppfølging av Sammen mot kreft – Nasjonal kreftstrategi 2013–2017 Sammen mot kreft og Nasjonal kreftstrategi 2018–2022 Leve med kreft.
Nasjonale handlingsprogrammer for kreftbehandling skal bidra til at det offentlige tilbudet i kreftomsorgen blir av god kvalitet og likeverdig over hele landet.
De nasjonale onkologiske faggruppene tilsluttet Onkologisk Forum hadde tidligere utviklet behandlingsveiledere for ulike krefttyper. Da arbeidet med de nasjonale handlingsprogrammene på kreftområdet startet i 2006, tok Helsedirektoratet utgangspunkt i disse.
Bakgrunn og arbeidsprosess ved 1. utgave (IS-1907)
24.06.11–18.12.13
Det nasjonale handlingsprogrammet med retningslinjer for diagnostikk, behandling og oppfølging av testikkelkreft ble første gang publisert 24. juni 2011 (IS-1907). Det ble skrevet av et arbeidsutvalg fra Norsk Urologisk Cancergruppe (NUCG), som utgjør Norsk testikkelkreftgruppe, og bestod av følgende personer:
Gustav Lehne, overlege dr.med, Avdeling for kreftbehandling, Oslo Universitetssykehus, Radiumhospitalet (leder)
Anders Angelsen, overlege dr.med, Avdeling for urologisk kirurgi, St. Olavs Hospital
Carl Wilhelm Langberg, overlege dr.med, Avdeling for kreftbehandling, Oslo Universitetssykehus, Ullevål
Olav Dahl, professor dr.med, Seksjon for onkologi, Institutt for indremedisin, Universitetet i Bergen og Kreftavdelingen, Haukeland universitetssykehus, Bergen
Roy Bremnes, professor dr.med, Kreftavdelingen, Universitetssykehuset Nord-Norge og Institutt for Klinisk Medisin, Universitetet i Tromsø
Torgrim Tandstad, lege, Kreftavdelingen, St. Olavs Hosptial
Rolf Wahlqvist, seksjonsoverlege dr.med, Urologisk avdeling, Oslo Universitetssykehus, Aker
Sophie Dorothea Fosså, professor dr.med, Avdeling for klinisk kreftforskning, Oslo Universitetssykehus, Radiumhospitalet
Hege Sagstuen Haugnes, lege førsteamanuensis PhD, Kreftavdelingen, Universitetssykehuset i Nord-Norge og Institutt for Klinisk Medisin, Universitetet i Tromsø
Karol Axcrona, overlege dr.med, Urologisk avdeling, Oslo Universitetssykehus, Radiumhospitalet
Forsker Lene Juvet, Nasjonalt kunnskapssenter for helsetjenesten bisto gruppen i arbeidet.
Utkast til retningslinjer var til behandling i Norsk Urologisk Cancergruppe og ble sendt på høring til brukerorganisasjoner under Kreftforeningen, de regionale helseforetakene og Den norske legeforening.
Bakgrunn og arbeidsprosess ved 2. utgave (IS-2119)
19.12.13–02.09.15
Retningslinjene ble i 2013 oppdatert av en gruppe bestående av følgende fagpersoner:
Torgrim Tandstad, overlege PhD, Kreftklinikken, St. Olavs Hospital (leder)
Olav Dahl, professor dr. med, Seksjon for onkologi, Institutt for indremedisin, Universitetet i Bergen og Kreftavdelingen, Haukeland universitetssykehus
Carl Wilhelm Langberg, overlege dr. med, Avdeling for kreftbehandling, Oslo Universitetssykehus
Rolf Wahlqvist, seksjonsoverlege dr. med, Urologisk avdeling, Oslo Universitetssykehus
Hege Sagstuen Haugnes, overlege PhD, Kreftavdelingen, Universitetssykehuset i Nord-Norge og Institutt for Klinisk Medisin, Universitetet i Tromsø
Jan Oldenburg, overlege dr. med, Avdeling for kreftbehandling, Oslo Universitetssykehus
Anders Angelsen, prof./overlege dr. med. Institutt for kreftforskning og molekylærmedisin, NTNU / Avdeling for urologisk kirurgi, St. Olavs Hospital
Marianne Brydøy, overlege dr. med., Kreftavdelingen, Haukeland universitetssykehus
Gruppen leverte 30. september 2013 utkast til oppdatering av retningslinjene. Helsedirektoratet ferdigstilte i samarbeid med forfatterne handlingsprogrammet i desember 2013.
Bakgrunn og arbeidsprosess ved 3. utgave (IS-2365)
03.09.15–14.04.2021
Fra 1. mai 2015 ble Pakkeforløp for testikkelkreft innført. De tidligere forløpstidene i handlingsprogram for testikkelkreft ble i denne utgaven erstattet av de nye tidene i Pakkeforløp for testikkelkreft.
Bakgrunn og arbeidsprosess ved 4. utgave (IS-2983)
15.04.2021
Denne utgaven av Nasjonalt handlingsprogram for diagnostikk, behandling og oppfølging av testikkelkreft er utarbeidet av arbeidsgruppen for Nasjonalt handlingsprogram for testikkelkreft i samarbeid med SWENOTECA-gruppen.
Medlemmer av handlingsprogramarbeidgruppen som har utarbeidet denne versjonen av handlingsprogrammet:
- Torgrim Tandstad, lege, PhD, onkolog, Kreftklinikken, St. Olavs Hospital HF (leder)
- Hege Sagstuen Haugnes, lege, onkolog, Kreftavdelingen, Universitetssykehuset i Nord-Norge HF
- Dag Halvorsen, lege, urolog, Avdeling for urologi, St. Olavs Hospital HF
- Ása Karlsdóttir, lege, onkolog, Kreftavdelingen, Helse Bergen HF, Haukeland universitetssykehus
- Carl W. Langberg, lege, PhD, onkolog, Kreftavdelingen, Oslo Universitetssykehus HF
- Helene Francisca Stigter Negaard, lege, PhD, onkolog, Kreftavdelingen, Oslo Universitetssykehus HF
- Rolf Wahlqvist, lege, urolog, Avdeling for urologi, Oslo Universitetssykehus HF
I tillegg har følgende fagpersoner bidratt i arbeidet:
- Signe Melsen Larsen, lege, urolog, Avdeling for urologi, Oslo Universitetssykehus HF
- Bjarte Almås, lege, urolog, Avdeling for urologi, Helse Bergen HF, Haukeland universitetssykehus
- Olav Dahl, lege, PhD, onkolog, Kreftavdelingen, Helse Bergen HF, Haukeland universitetssykehus og professor, Universitetet i Bergen, Bergen
- Erik Rud, lege, PhD, Avdeling for radiologi og nukleærmedisin, Oslo Universitetssykehus HF, når det gjelder delen om bildediagnostikk
- Monika Eidem, lege, Kreftklinikken ved St. Olavs Hospital HF, når det gjelder delen om radioterapi.
Habilitet
Alle gruppens medlemmer ble i forbindelse med arbeidet bedt om å oppgi potensielle interessekonflikter. Ingen interessekonflikter ble oppgitt. Helsedirektoratet har vurdert arbeidsgruppens medlemmer som habile i forhold til utarbeiding av utkast til nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av testikkelkreft.
Ressursmessige konsekvenser
Nye behandlingsmetoder mot kreft kan medføre økte kostnader, men kan også føre til reduserte kostander i andre deler av pasientbehandlingen. Anbefalingene i dette handlingsprogrammet kan derfor medføre omdisponering av ressurser i de regionale helseforetakene.
Oppdatering av retningslinjene
Utviklingen av ny behandling på kreftområdet er rask. Det kan være behov for å endre retningslinjene fordi det er behandling som er «utdatert» eller at det er behov å starte en prosess med vurdering av aktuell ny og kostbar kreftbehandling.
Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av testikkelkreft vil vurderes årlig og om nødvendig oppdateres etter følgende prosess:
- Norsk testikkelkreftgruppe melder fra til Helsedirektoratet om behov for å endre retningslinjene
- Oppdateringen utføres av en redaksjonskomité som består av representanter fra fagmiljøet, Nasjonalt kunnskapssenter for helsetjenester og Helsedirektoratet.
- De oppdaterte retningslinjene vil foreligge på www.helsedirektoratet.no
Appendix
I Prognosegrupper etter IGCCCG
Sist faglig oppdatert: 15.04.2021
| Non-seminom | Seminom |
|---|---|
|
God prognose |
|
|
Primærlokalisasjon: Testis eller retroperitoneum |
Alle primærlokalisasjoner |
|
Intermediær prognose |
|
|
Primærlokalisasjon: Testis eller retroperitoneum |
Ikke-pulmonale viscerale metastaser (for eksempel lever, skjelett, hjerne) |
|
Dårlig prognose |
|
|
Primærtumor i mediastinum |
Ingen seminom med dårlig prognose |
II Modifisert Royal Marsden Hospital Stadium
Sist faglig oppdatert: 15.04.2021
| CS I | Ingen tegn til metastaser | |
| CS Mk+ | Tumormarkører AFP/ß-hCG vedvarende forhøyet (ikke fallende iht. halveringstid), men ingen påvist makroskopisk metastatisk sykdom | |
| CS II |
Metastaser begrenset til intraabdominale lymfeknuter: A Maksimal transversal diameter < 2 cm B Maksimal transversal diameter 2–5 cm C Maksimal transversal diameter > 5–10 cm D Maksimal transversal diameter > 10 cm |
|
| CS III |
Metastaser til supradiafragmatiske lymfeknuter For abdominale lymfeknuter: 0: Ingen metastaser; A–D: Som CS II. |
|
| CS IV |
Ekstralymfatiske metastaser For abdominale lymfeknuter: |
|
III Flytskjema: RPLND av seminom i klinisk stadium IIA–IIB (≤ 3 cm)
Sist faglig oppdatert: 15.04.2021
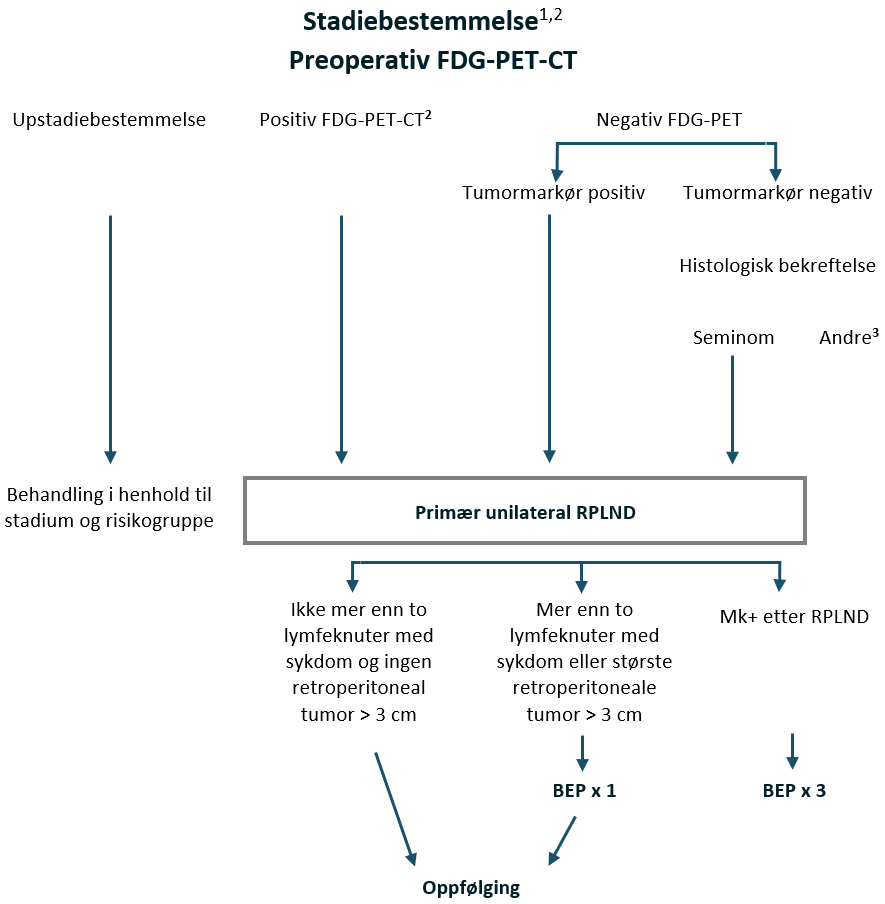
1 For riktig stadiebestemmelse av pasienter i CS IIA, se Stadiebestemmelseundersøkelser
2 Ikke mer enn to lymfeknuter eller sykdom utenfor unilateralt templat for RPLND
3 Overvåkning hvis benign, behandling iht. stadium og risikogruppe hvis non-seminom
IV Flytskjema: Metastatisk seminom med god prognose, unntatt klinisk stadium IIA–IIB (≤ 3 cm) egnet for kirurgi
Sist faglig oppdatert: 15.04.2021
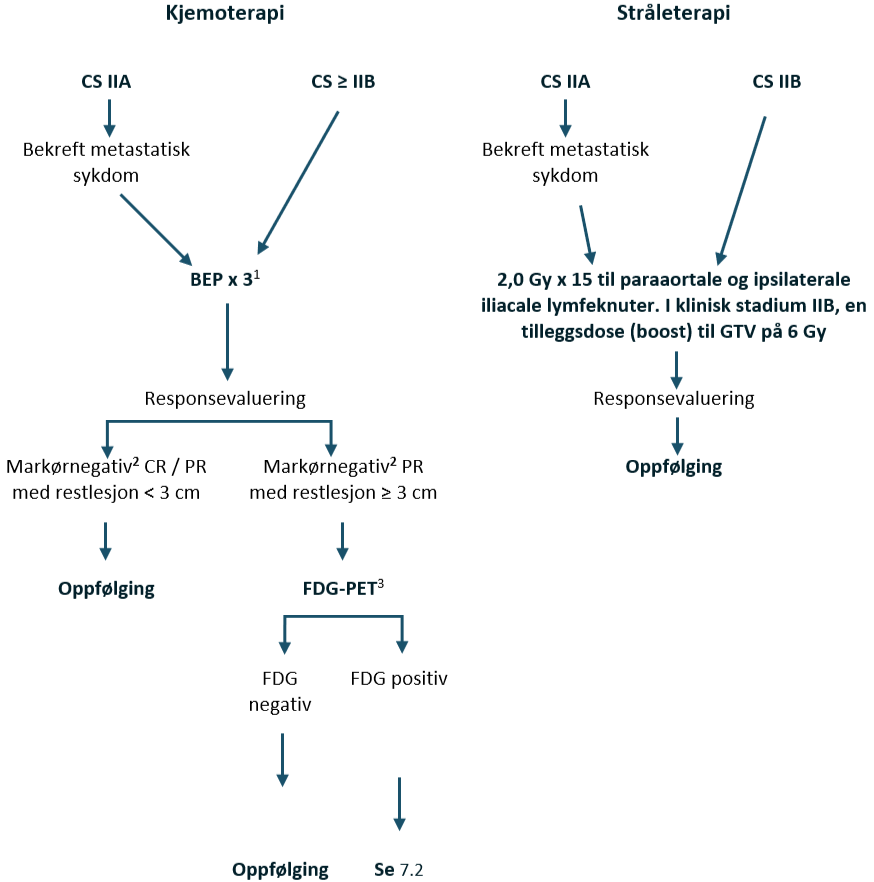
1 PEI x 3 eller EP x 4 er valgfri behandling ved kontraindikasjoner mot bleomycin
2 Ved positive markører etter kjemoterapi, diskuter innenfor SWENOTECA
3 Ikke tidligere enn 9 uker etter dag 1 av den siste kuren med cellegift
V Flytskjema: Metastatisk seminom med intermediær prognose
Sist faglig oppdatert: 15.04.2021
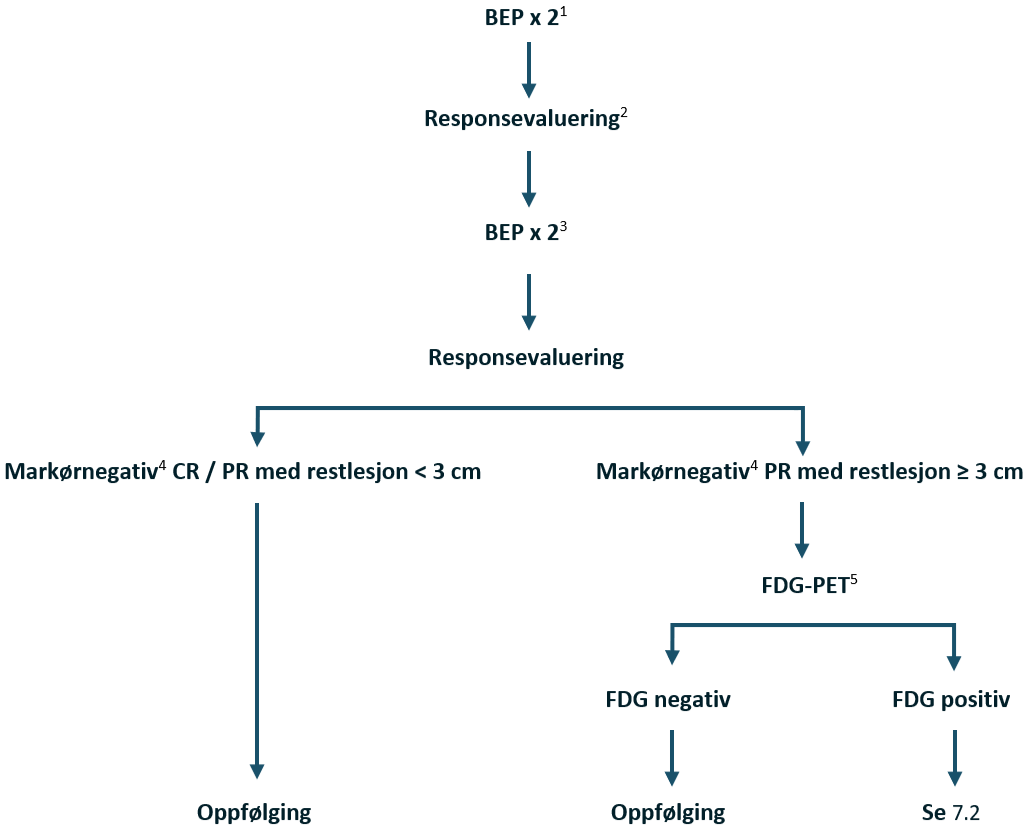
1 Hos pasienter med kontraindikasjoner mot bleomycin eller hjernemetastase bør PEI velges
2 Ved markørnegativ sykdom og mangel på radiologisk respons etter to kurer med kjemoterapi,
bør muligheten for teratom vurderes og det bør utføres en biopsi
3 Maksimal kumulativ dose bleomycin er 300 000 enheter
4 Ved positive markører etter kjemoterapi, diskuter innenfor SWENOTECA
5 Ikke tidligere enn 9 uker etter dag 1 av den siste kuren med cellegift
VI Flytskjema: Non-seminom CS IIA Mk- ved diagnosetidspunkt/stadiebestemmelse
Sist faglig oppdatert: 15.04.2021
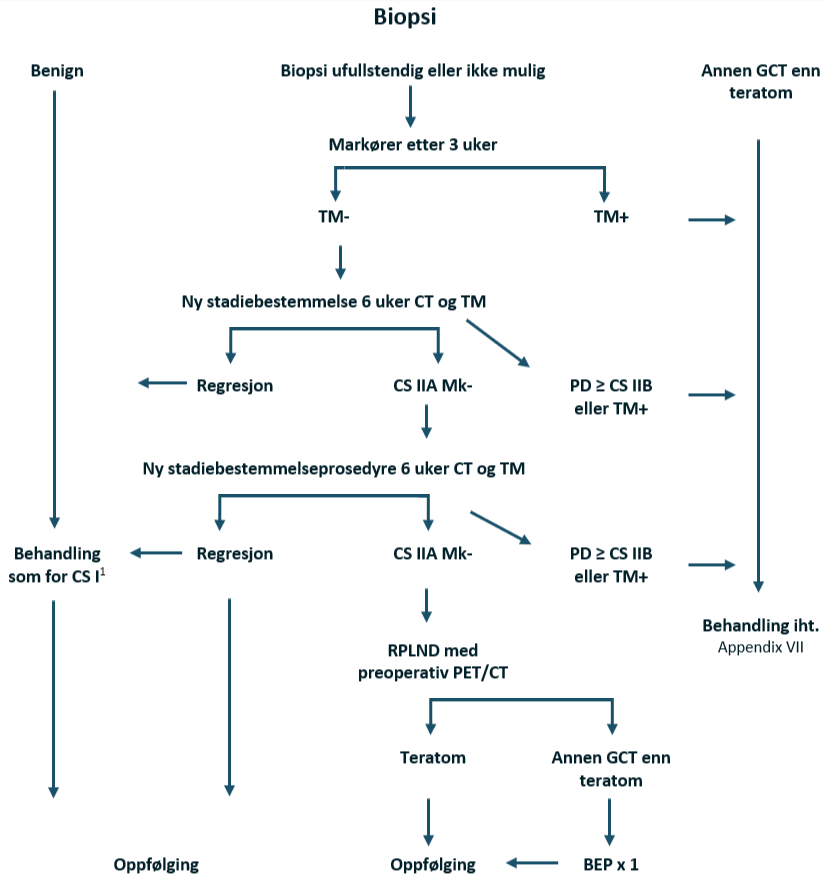
1 Adjuvant kjemoterapi gis ikke senere enn 15 uker etter orkiektomi
VII Flytskjema: Metastatisk non-seminom med god prognose, unntatt CS IIA Mk-
Sist faglig oppdatert: 15.04.2021
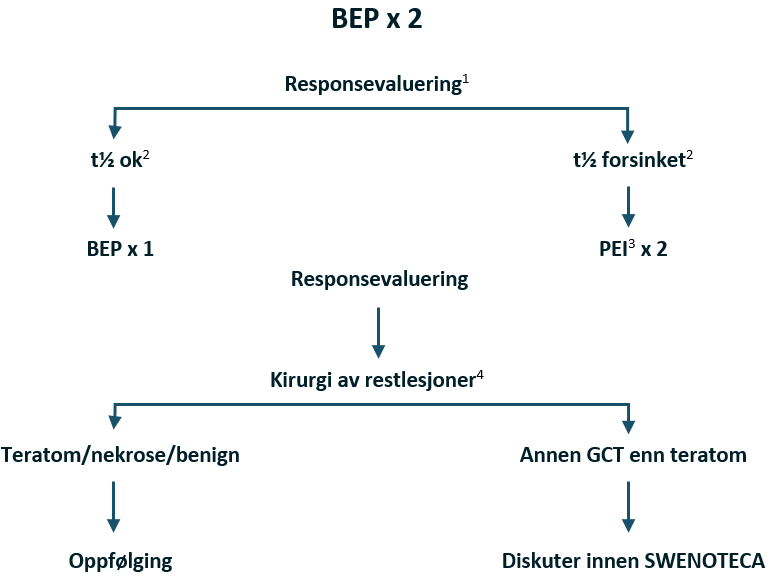
1 Hos pasienter uten forhøyede markører før oppstart av kjemoterapi og radiologisk regresjon < 25 % bør kirurgi vurderes hos pasienter med hovedsakelig teratom i testikkelen
2 Markør t½ ok: AFP ≤7 dager, ß-hCG ≤3 dager, markør t½ forsinket: AFP ≤7 dager, ß-hCG ≤3 dager, progresjon av markør: Reevaluer behandlingsresistent metastase (hjerne/skjelett) eller ny primærsvulst, vurder kirurgi
3 Ved initial behandling med PEI: Intensiver til TIPS
4 Alltid kirurgi av restsykdom ved non-seminom om mulig
VIII Flytskjema: Metastatisk non-seminom med intermediær og dårlig prognose, unntatt ikke-pulmonal visceral metastase eller primær mediastinal tumor1
Sist faglig oppdatert: 15.04.2021
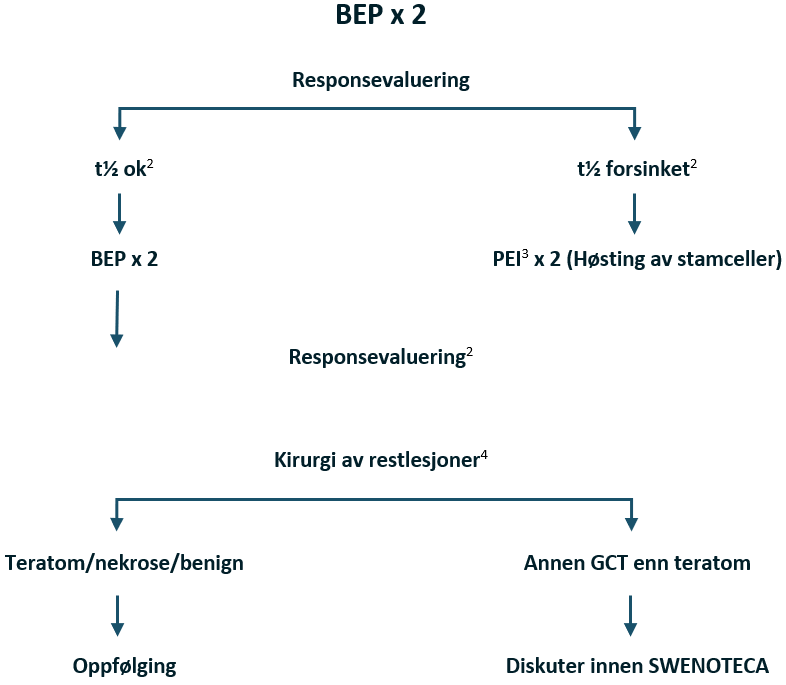
1 Markører for intermediær prognose: AFP ≥1000 – 10 000 ng/ml eller ß-hCG ≥5000–50 000 IE/L eller
LDH ≥1,5 – 10 x øvre normalgrense;
Markør for dårlig prognose: AFP >10 000 ng/ml eller ß-hCG >50 000 IU/L eller LDH >10 x øvre normalgrense;
2 t½ ok: AFP ≤7 dager, ß-hCG ≤3 dager, markør t½ forsinket: AFP ≤7 dager, ß-hCG ≤3 dager, progresjon av markør: Reevaluer for behandlingsresistent metastase (hjerne/skjelett) eller ny primærsvulst; progresjon: Diskuter innen SWENOTECA
3 Ved initial behandling med PEI: Intensiver til TIPS
4 Alltid kirurgi av restsykdom ved non-seminom om mulig, også kirurgi ved PR TM+ med fallende/stabile markører
IX Flytskjema: Metastatisk non-seminom med dårlig prognose med ikke-pulmonal visceral metastase eller primær mediastinal tumor
Sist faglig oppdatert: 15.04.2021
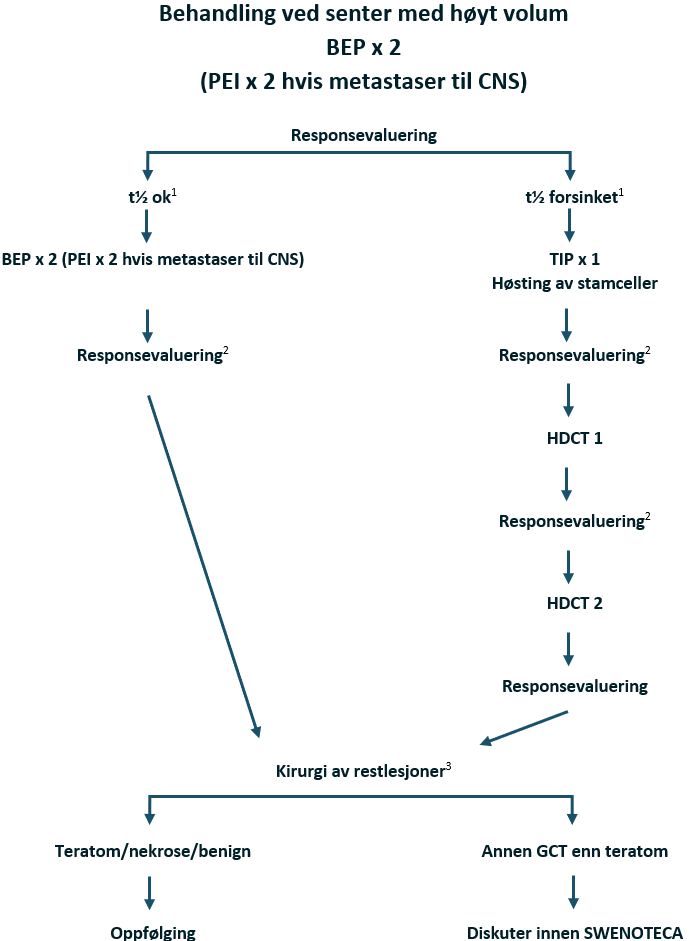
1 t½ ok: AFP ≤7 dager, ß-hCG ≤3 dager, markør t½ forsinket: AFP ≤7 dager, ß-hCG ≤3 dager, progresjon av markør: Reevaluer for behandlingsresistent metastase (hjerne/skjelett) eller ny primærsvulst; progresjon: Diskuter innen SWENOTECA
2 Progresjon: Diskuter innen SWENOTECA
3 Alltid kirurgi av restsykdom ved non-seminom om mulig, også kirurgi ved PR TM+ med fallende/ stabile markører
X Flytskjema: Ekstragonadal germinalcellekreft (EGCT)
Sist faglig oppdatert: 15.04.2021
.png)
GCNIS
XI Flytskjema: Hjernemetastaser ved diagnose
Sist faglig oppdatert: 15.04.2021
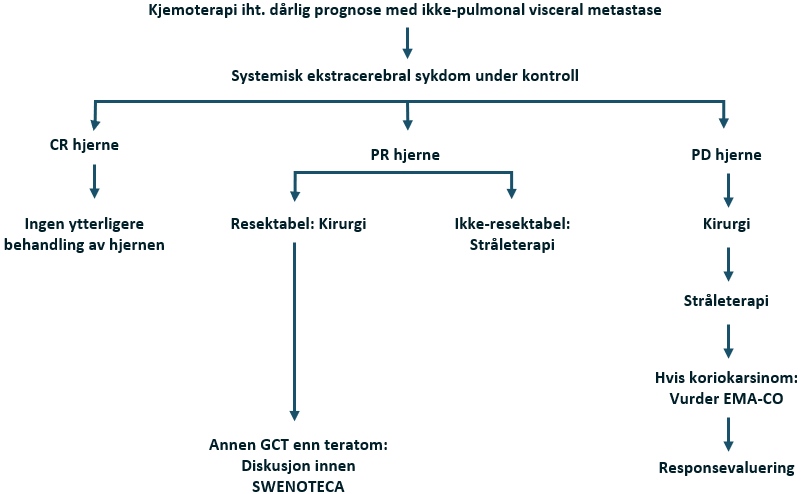
Stråleterapi
Helhjernestråling: 1,8 Gy x 22 (totaldose 39,6 Gy) med SIB 2,45 Gy x 22 (totaldose 53,9 Gy).
Stereotaktisk strålebehandling: Få (maks 4) og mindre metastaser.
XII Flytskjema: Hjernemetastaser ved residiv etter CR
Sist faglig oppdatert: 15.04.2021
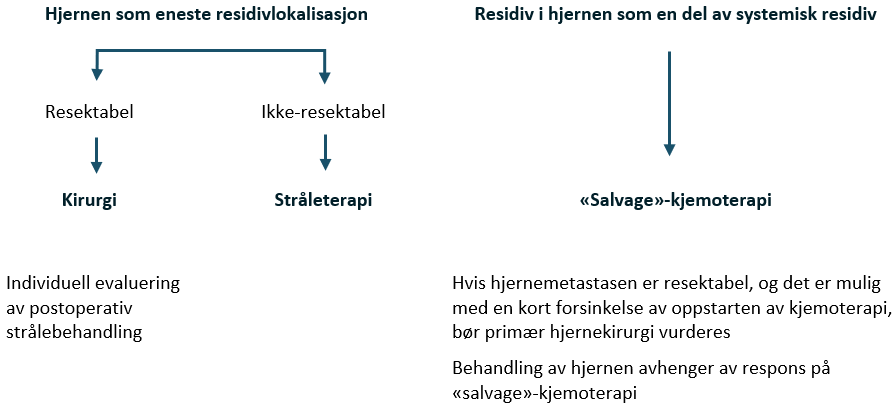
Stråleterapi
Helhjernestråling: 1,8 Gy x 22 (totaldose 39,6 Gy) med SIB 2,45 Gy x 22 (totaldose 53,9 Gy).
Stereotaktisk strålebehandling: Få (maks 4) og mindre metastaser.
XIII Flytskjema - «Salvage»-behandling ved initial metastatisk sykdom
Sist faglig oppdatert: 15.04.2021
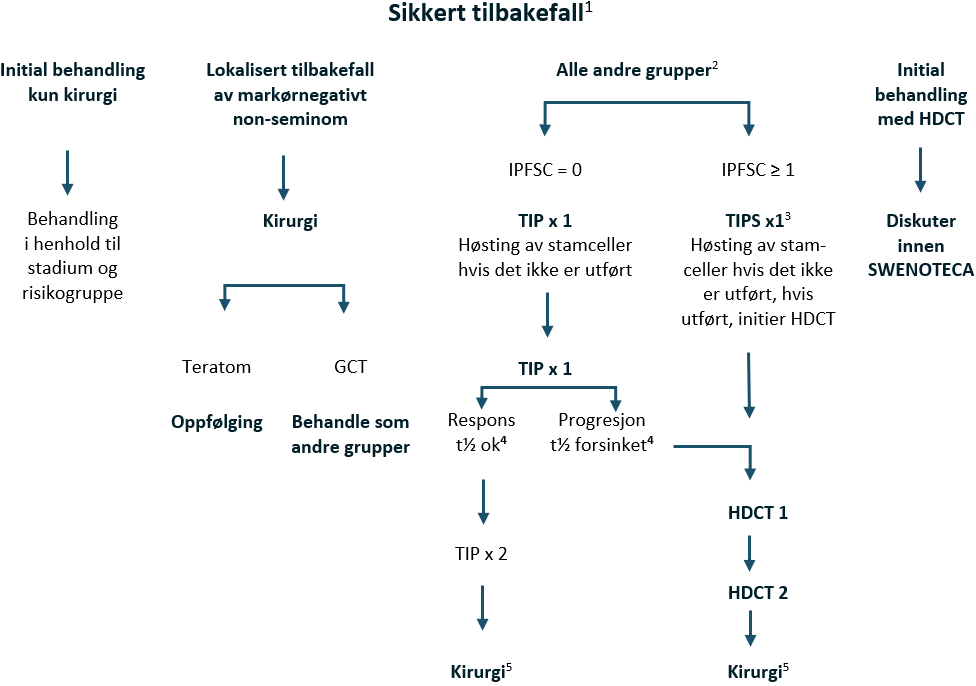
1 Markør positiv, biopsi verifisert eller klinisk sikkert
2 Ved små lokaliserte seminomresidiv kan strålebehandling være en behandlingsmulighet
3 Hvis pasienten har fått flere kurer TIP som initial behandling, kan andre kurer velges som mobilisering
4 Ved non-seminom
5 Alltid kirurgi av restsykdom ved non-seminom om mulig, ved seminom biopsi av PET-positive eller voksende lesjoner (Dersom funn av vital kreft, er kirurgi anbefalt behandling)
XIV Oppfølgingsplan for seminompasienter
Sist faglig oppdatert: 15.04.2021
XV Oppfølgingsplan for pasienter med non-seminom stadium I, overvåkning
Sist faglig oppdatert: 15.04.2021
XVI Oppfølgingsplan for pasienter med non-seminom stadium I, adjuvant BEP
Sist faglig oppdatert: 15.04.2021
XVII Oppfølgingsplan for pasienter med non-seminom etter behandling for metastatisk eller tilbakevendende sykdom
Sist faglig oppdatert: 15.04.2021
XIX MR-protokoll
Sist faglig oppdatert: 15.04.2021
Anbefalt protokoll for MR av abdomen ved oppfølging av pasienter med testikkelkreft
Generell bildeprotokoll for utstyr med magnetfelt på 1,5T og 3T. Undersøkelsen utføres etter minst fire timers faste.
Foruten en koronar lokalisator (helst STIR-eller HASTE-basert) utføres det transversale T2-vektede (helst «konvensjonelle» TSE-/FSE-sekvenser eller HASTE-sekvenser avhengig av utstyr) T1-vektede (helst DIXON-basert 3D) pulssekvenser med maksimal 4 mm snittykkelse og maksimum 10 % avstand mellom snittene fra diafragma til symphysis pubica. Den spatielle oppløsningen bør maksimeres med det spesifikke utstyret som brukes.
Endelig utføres transversale (multi-shot) EPI-baserte DWI-sekvenser med en maksimal snittykkelse på 6 mm med samme dekning med b-verdier på 0 og 800 s/mm2 og inkludert et ADC-kart.
Det er viktig at undersøkelsene utføres og tolkes på et senter med både kompetanse, et tilstrekkelig kontinuerlig antall abdominale MR samt tilgjengelighet på teknologer og radiologer som er kjent med det relevante mønsteret av tumorresidiv i denne pasientgruppen for å legge vekt på de riktige aspektene ved undersøkelsen både med hensyn til forberedelse, gjennomføring og tolkning. Det er viktig at undersøkelsene er fokusert på retroperitoneum siden dette er den viktigste lokalisasjonen for tilbakefall i denne pasientgruppen.
|
MR abdomen SWENOTECA |
||||
|---|---|---|---|---|
|
Sekvens |
STIR/HASTE Lokalisator |
TSE/FSE |
T1 «spoiled» gradientekko |
T2/EPI b0_b800 |
|
Vekting |
T1 eller 2 |
T2 |
T1 |
DWI |
|
Orientering |
Cor |
Ax |
Ax |
Ax |
|
TR /TE (ms) |
4–5000/82 |
4–6000/100 |
165/2 i motsatt fase |
5600/60 |
|
Synsfelt (mm) |
480x275 |
320–400x275 |
320–400x275 |
320–400x275 |
|
Matrise |
256/128 |
512/215 |
256/136 |
192/156 |
|
Tykkelse/dst (mm) |
5/1 |
Min |
Min |
5/1 |
|
NEX/NSA |
1 |
1 |
1 |
4 |
|
Antall seksjoner |
||||
|
Dekning |
Hele abdomen |
Diafragmasymfyse |
Diafragmasymfyse |
Diafragmasymfyse |
|
Skannetid (min:sek) |
||||
Ytterligere behandling
☐ Ingen
☐ Cellegift (type og antall kurer: ____________________________ )
☐ Strålebehandling
☐ Operasjoner i tillegg til fjerning av testikkelen _____________________________
Dato for siste kontroll: __________________ Sykehus: ______________________
Behandlende lege: __________________ Telefon: ______________________
Du har vært til avsluttende sykehuskontroll etter tidligere behandling for testikkelkreft. Det er svært liten risiko for tilbakefall av sykdommen. Dette skrivet bør tas med ved senere kontakter med helsevesenet.
Det er en økt forekomst av ny svulst i gjenværende testikkel og det er viktig med regelmessig selv-undersøkelse. Menn som tidligere er behandlet med cellegift og/eller strålebehandling har en økt risiko for høyt blodtrykk, overvekt, forhøyet kolesterol og hjerte-karsykdommer. Derfor er det lurt å avstå fra røyking, forsøke å unngå overvekt og trene regelmessig. Dessuten kan en ny krefttype utvikles flere år etter behandling med cellegift og/eller strålebehandling. Vi anbefaler deg å kontakte fastlegen din hvis du opplever helseproblemer i fremtiden.
Du har også en risiko for lave nivåer av mannlige kjønnshormoner. Kjønnshormonnivåene dine bør kontrolleres hvis du opplever energiløshet eller manglende seksuell interesse/funksjon.
Menn behandlet med full kjemoterapi eller strålebehandling for metastatisk sykdom anbefales regelmessige helsekontroller (f.eks. hvert 2.–5. år) hos fastlegen:
- Blodtrykk, høyde og vekt
- Blodprøver som inkluderer fastende lipider (totalkolesterol, HDL- og LDL-kolesterol, triglyserider), glukose, HbA1c og kjønnshormoner (testosteron, SHBG, LH og FSH) ved symptomer på hypogonadisme
- Klinisk undersøkelse ved symptomer
- Initier bildebehandling eller henvis til spesialist ved mistanke om tilbakefall, sekundærkreft eller kontralateral testikkelkreft
Formålet med disse kontrollene er å forebygge, identifisere og muligens behandle komplikasjoner etter den gjennomgåtte kreftbehandlingen. Hvis det oppdages unormale verdier ved disse kontrollene, initieres det ytterligere oppfølging hos fastlegen.
XX BEP
Sist faglig oppdatert: 15.04.2021
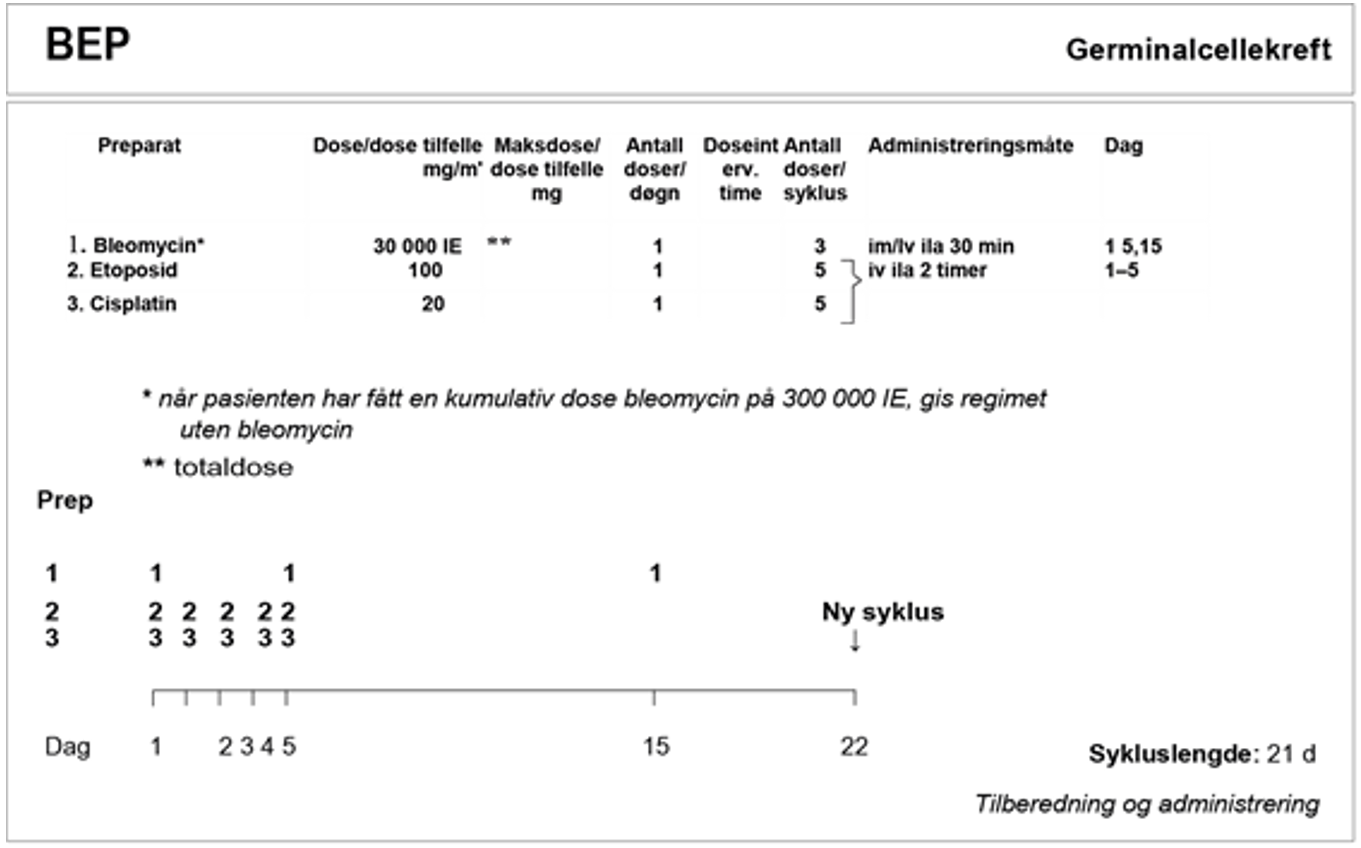
Spesielle tiltak
Cisplatin: S-kreatinin før hver syklusstart. Hvis patologisk, utføres iohexol-clearance. Cisplatin gis med forsert diurese.
CAVE! aminoglykosid skal ikke gis under eller en måned etter cisplatinbehandling.
Bleomycin: hvis toksisk reaksjon på bleomycintilførsel (feber, frysninger) gis steroider som Deltison 25 mg per os eller 3-4 mg Betapred. Heretter gis steroider profylaktisk før bleomycin.
|
Benmargstoksisitet |
|||||
|---|---|---|---|---|---|
| Nøytrofil x 109/L | TPK x 109/L | Preparat, % av full dose | Tiltak | ||
| 1 | 2 | 3 | Gi behandling. G-CSF iht. lokale retningslinjer. Merk! - hvis TPK ca. 50, skal nadir være passert! Behandlingen utsettes i maksimalt 3 dager. Behandlingen kan imidlertid gis etterfulgt av G-CSF hvis situasjonen krever det! Behandlingen utsettes til TPK ≥ 50 |
||
| > 0.5 og <1,0 | ☐ 50 | 100 | 100 | 100 | |
| < 0,5 | ≥ 50 | ||||
| < 50 | |||||
| Nedsatt nyrefunksjon* | ||||
|---|---|---|---|---|
| Korrigert iohexol-clearence (ml/min/1.73 m2), normalverdi 80-125 for 18-50 år. | ||||
| 50-59 | 100 | 100 | 100 | Cisplatin gis kun i 4 dager |
| 40-49 | 50 | 100 | 100 | Cisplatin gis kun i 3 dager |
| < 40 | 0 | 100 | ** | Cisplatin erstattes med Karboplatin dosert etter Calverts formel AUC 7** |
| Korrigert iohexol-clearence (ml/min/1,73 m2), normalverdi 60-110 for 51-65 år. | ||||
| 40-49 | 50 | 100 | 100 | Cisplatin gis kun i 4 dager |
| < 40 | 0 | 100 | ** | Cisplatin erstattes med Karboplatin dosert etter Calverts formel AUC 7** |
| * Men, hvis nedsatt nyrefunksjon skyldes tumorobstruksjon, skal det gis full dose Cisplatin. Nefrostomi kan være nødvendig. **Totaldose Karboplatin, mg = 7 x (ukorrigert clearence ml/min + 25). Karboplatin gis kun dag 1! |
||||
| Merk Bleomycin: CAVE! Det foreligger fare for alvorlig pneumonitt. Vær observant på tegn på pneumonitt. Økt risiko ved høy akkumulert totaldose, nedsatt nyrefunksjon, eldre pasienter, høy O2-konsentrasjon i inhalert luft, tidligere eller samtidig strålebehandling mot thorax. |
||||
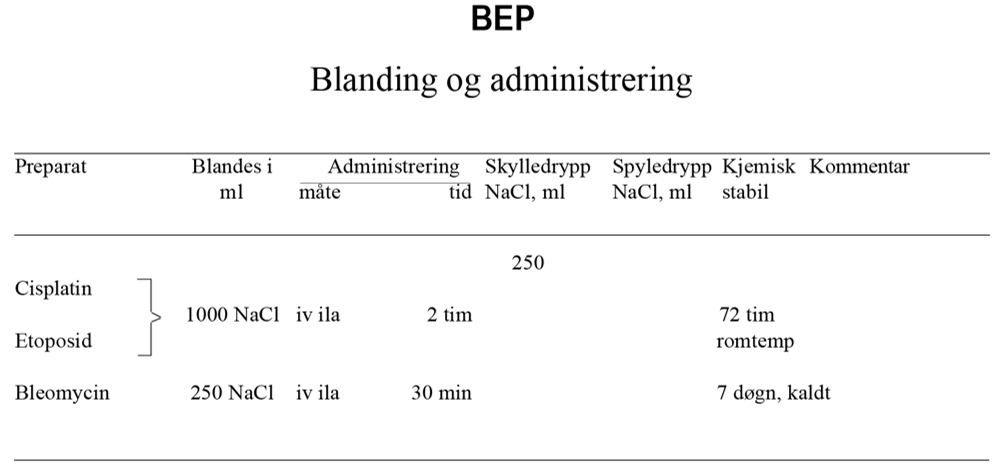

XXI EP
Sist faglig oppdatert: 15.04.2021
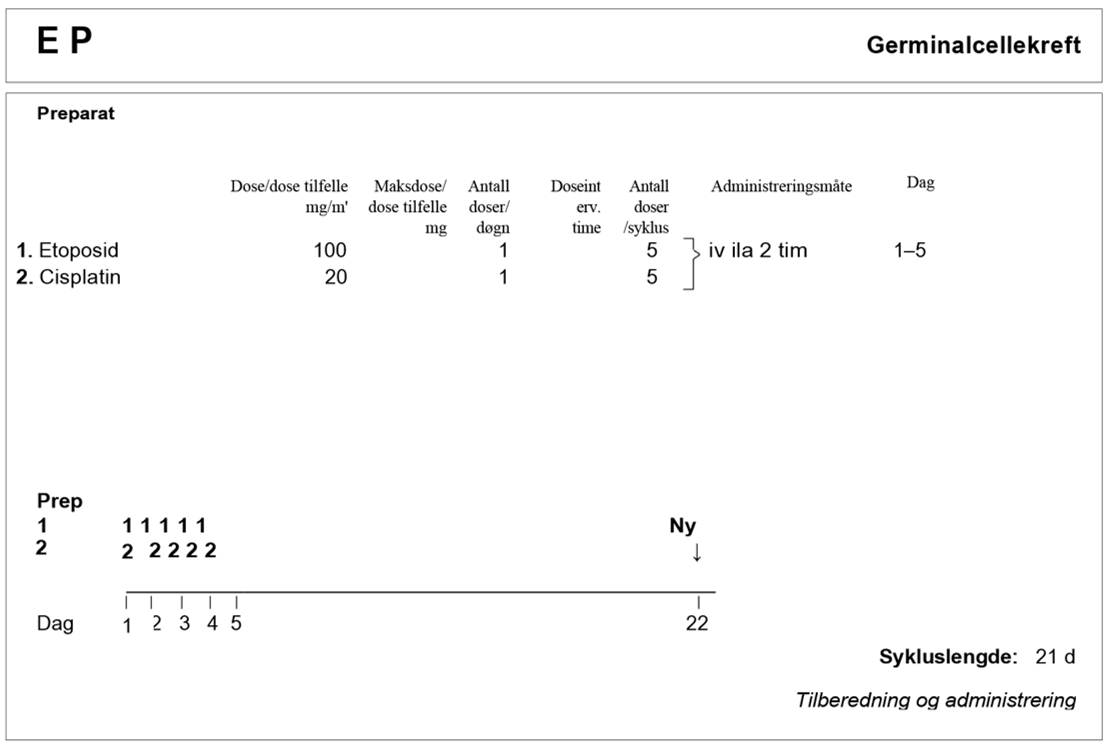
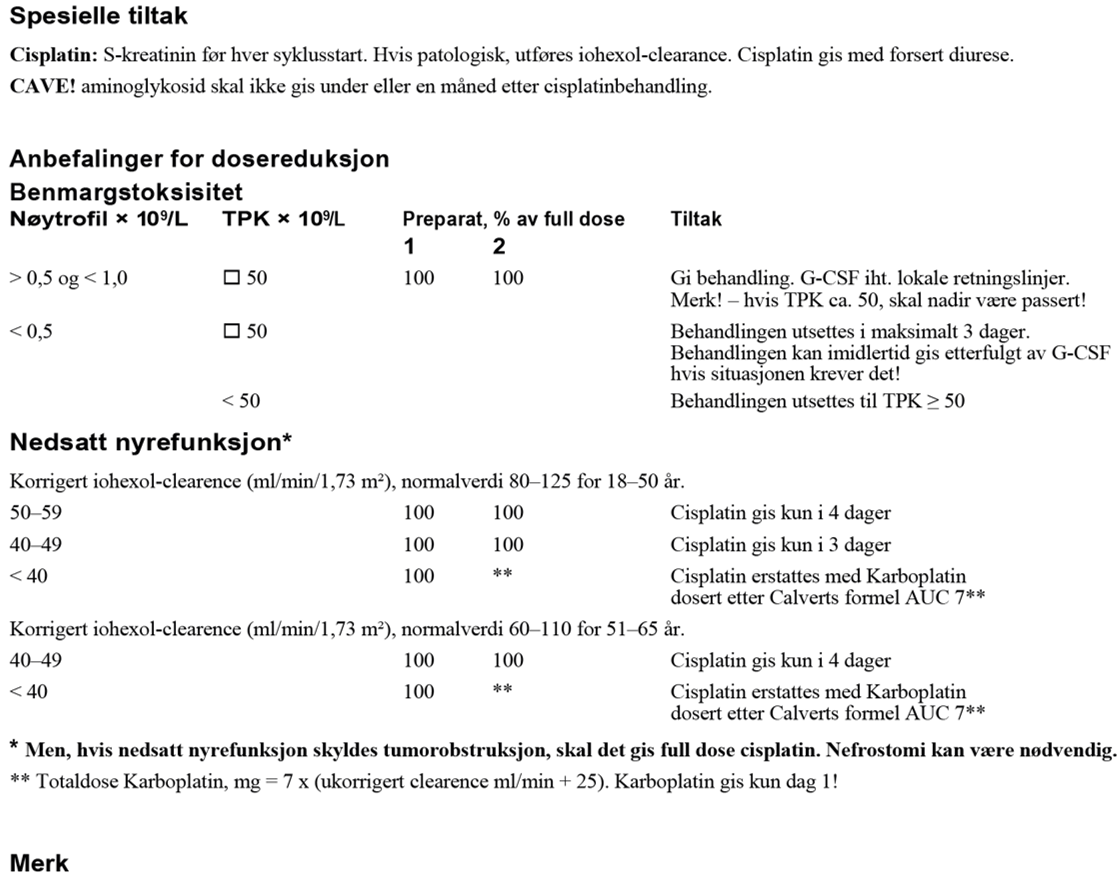


XXII Karboplatin
Sist faglig oppdatert: 15.04.2021
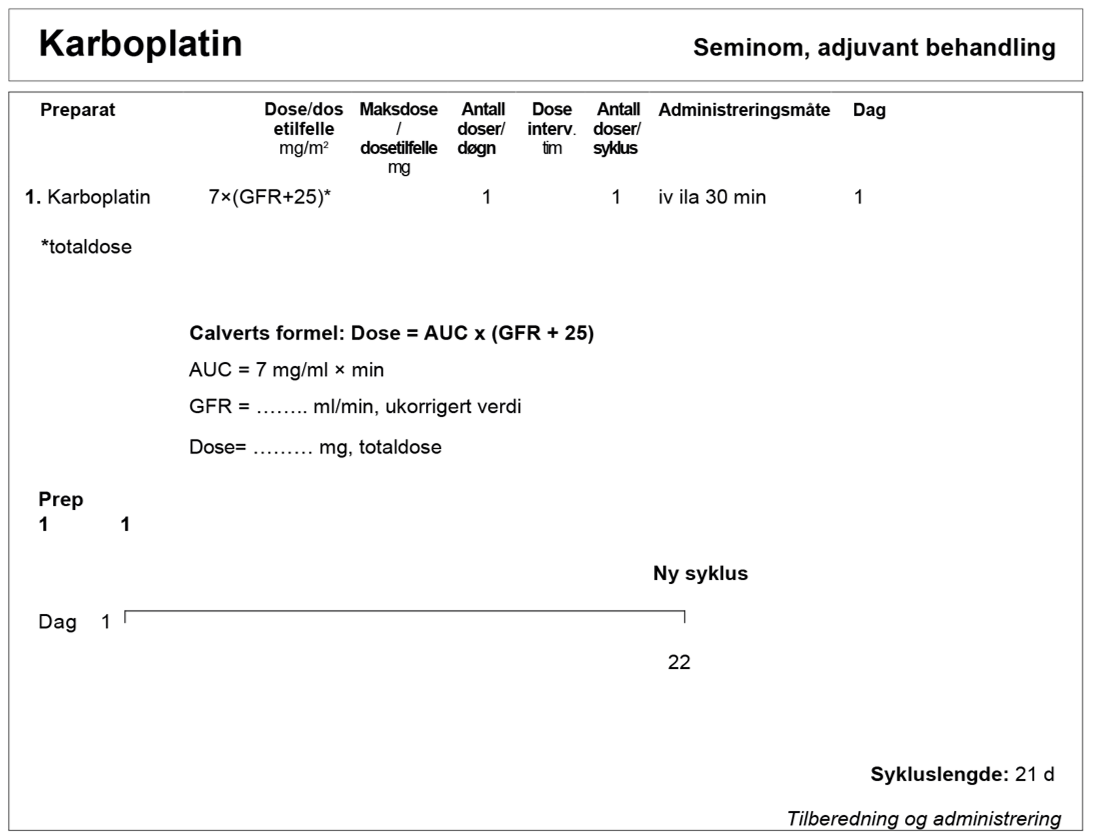


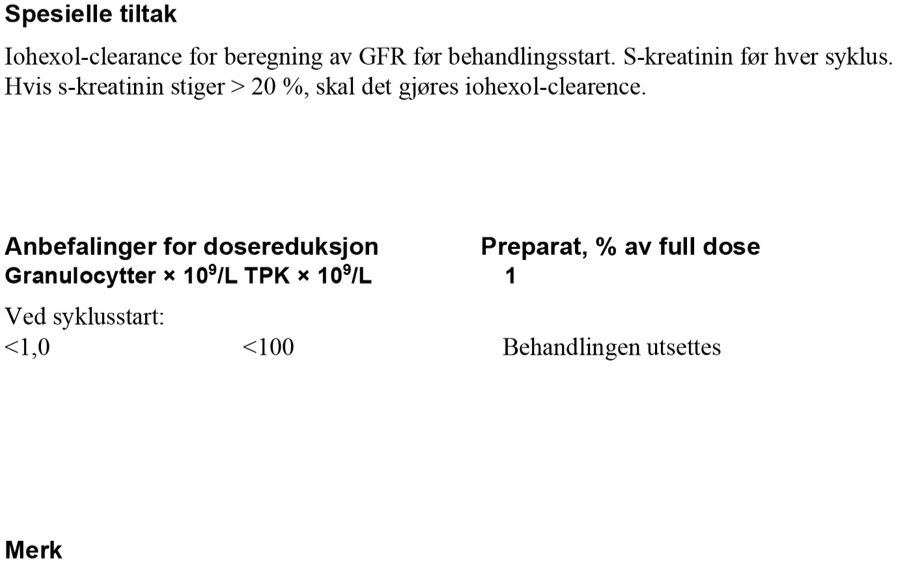
XXIII TIP
Sist faglig oppdatert: 15.04.2021
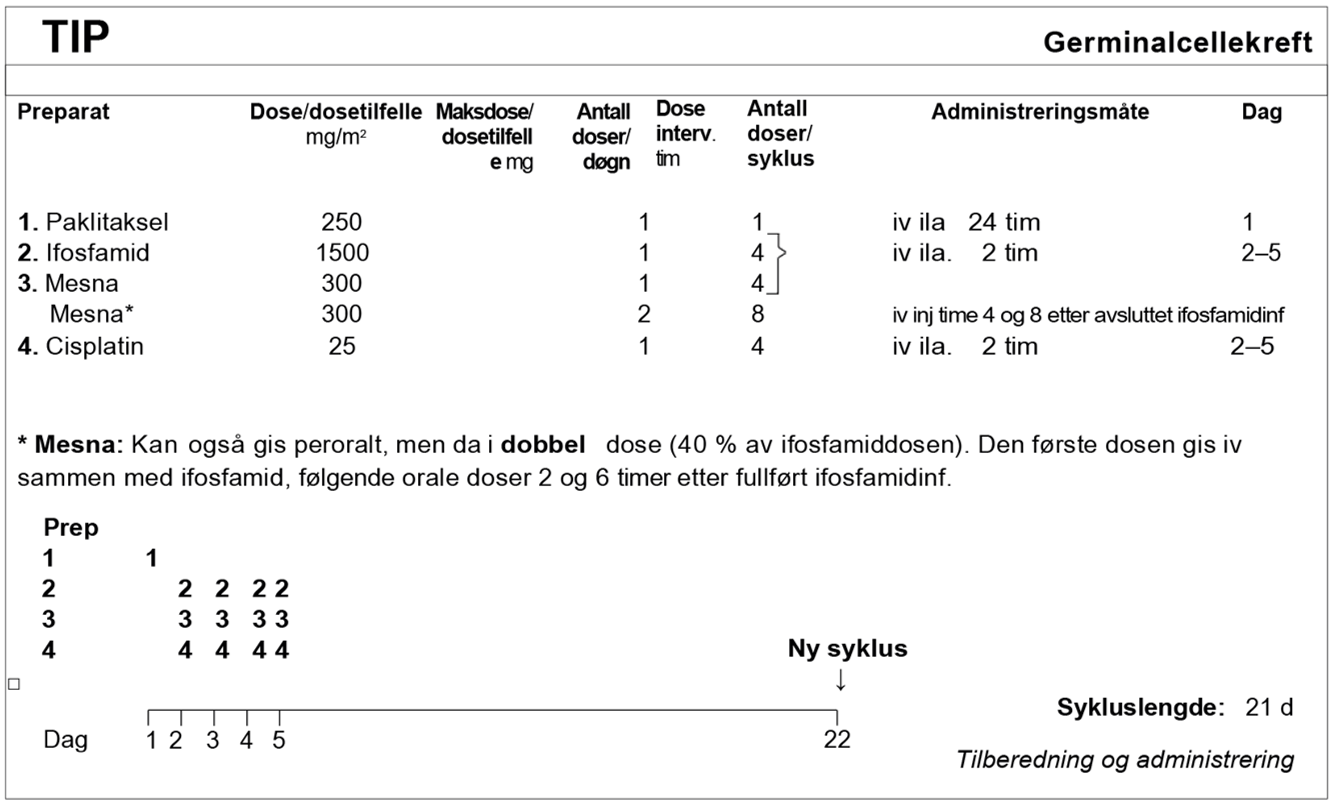


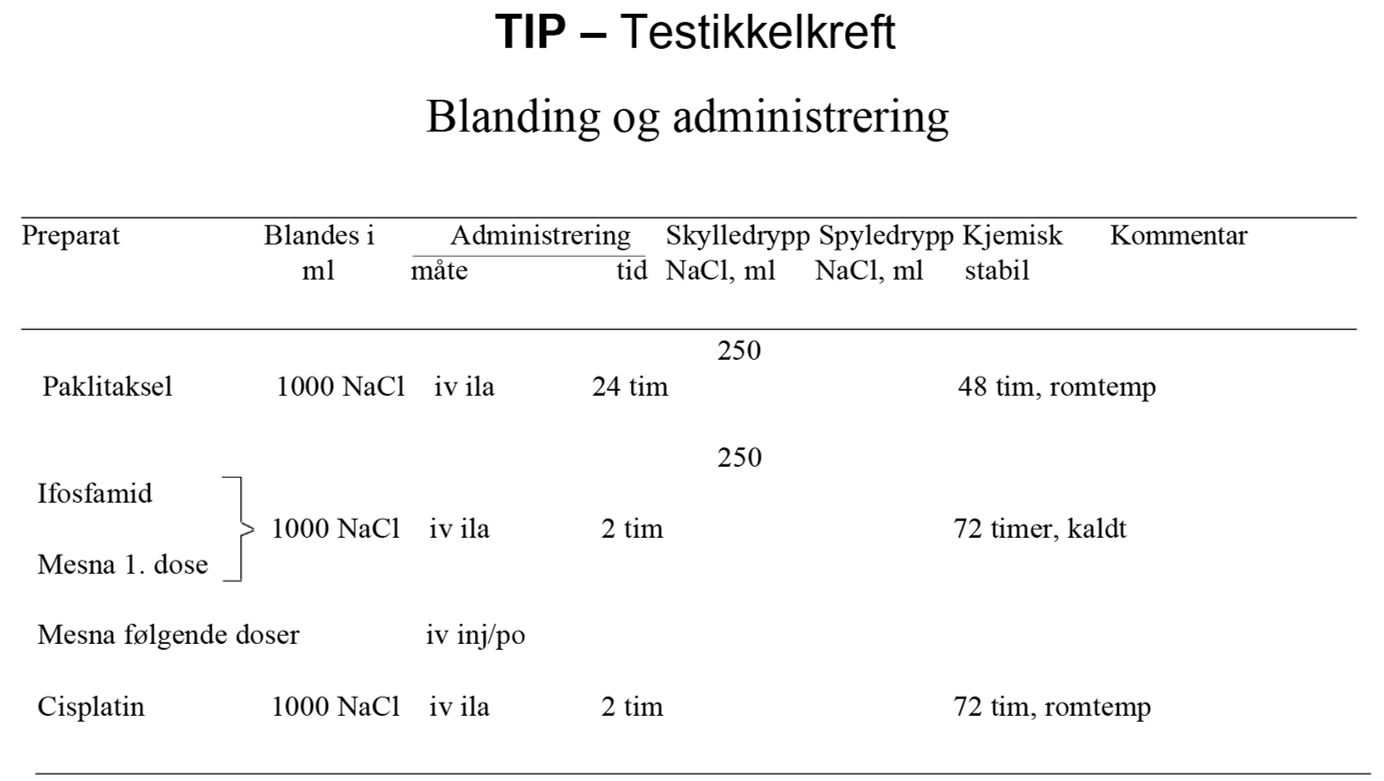

XXIV PEI
Sist faglig oppdatert: 15.04.2021
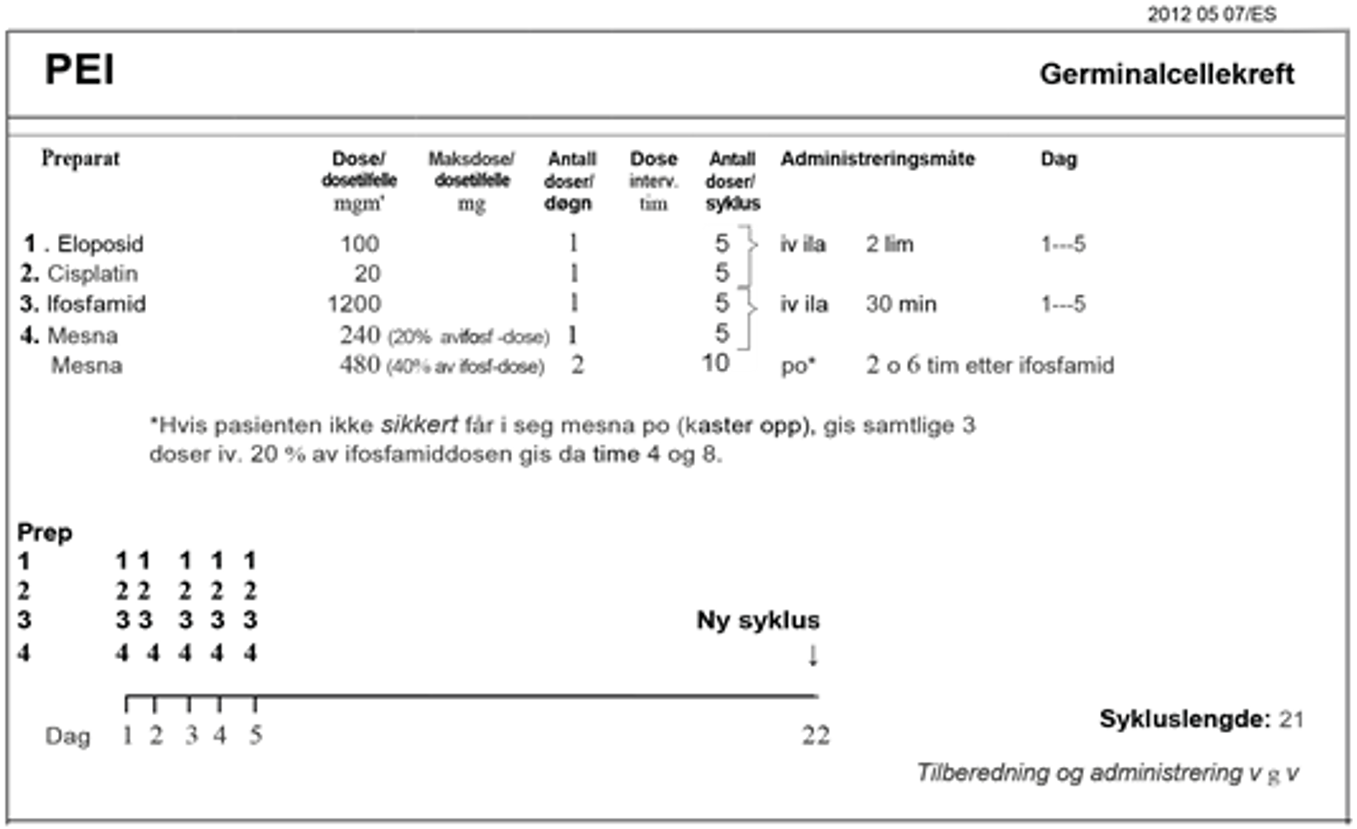
Spesielle tiltak
Cisplatin: S-kreatinin før hver syklusstart. Hvis patologisk, utføres iohexol-clearance. Cisplatin gis med forsert diurese.
CAVE! aminoglykosid skal ikke gis under eller en måned etter cisplatinbehandling.
Ifosfamid: Vær observant på cystittplager. Hematuri stiks ved behov. Hvis 3+ avbrytes ifosfamidbehandling.
| Benmargstoksisitet | |||||
|---|---|---|---|---|---|
| Nøytrofile >< 109/L | TPK >< 109/L |
Preparat, % av full dose | Tiltak | ||
| 1 | 2 | 3+4 |
Gi behandling. G-CSF iht. lokale retningslinjer. Merk! - hvis TPK ca. 50, skal nadir være passert! Behandlingen utsettes i maksimalt 3 dager. Behandlingen kan imidlertid gis etterfulgt av G-CSF hvis situasjonen krever det! Behandlingen utsettes til TPK ≥ 50. |
||
| > 0,5 og < 1,0 |
≥50 | 100 | 100 | 100 | |
| < 0,5 | ≥50 | ||||
| ≥50 | |||||
| Nedsatt nyrefunksjon* | ||||
|---|---|---|---|---|
| Korrigert iohexol-clearence (ml/min/1.73 m2), normalverdi 80-125 for 18-50 år. | ||||
| 50-59 | 100 | 100 | 100 | Cisplatin gis kun i 4 dager Cisplatin gis kun i 3 dager Ifosfamid og Mesna gis kun i 4 dager. Cisplatin erstattes med Karboplatin dosert etter Calverts formel AUC 7** Ifosfamid og Mesna gis kun i 4 dager |
| 40-49 | 100 | 100 | 100 | |
| <40 | 100 | 100 | ||
| Korrigert iohexol-clearance (ml/min/l, 73 m2), normalverdi 60-110 for 51-65 år. | ||||
| 40-49 | 100 | 100 | 100 |
Cisplatin gis kun i 4 dager |
| <40 | 100 | 100 | ||
| * Men, hvis nedsatt nyrefunksjon skyldes tumorobstruksjon, skal det gis full dose Cisplatin. Nefrostomi kan være nødvendig. **Totaldose Karboplatin, mg = 7 x (ukorrigert clearence ml/min + 25). Karboplatin gis kun dag 1! |
||||
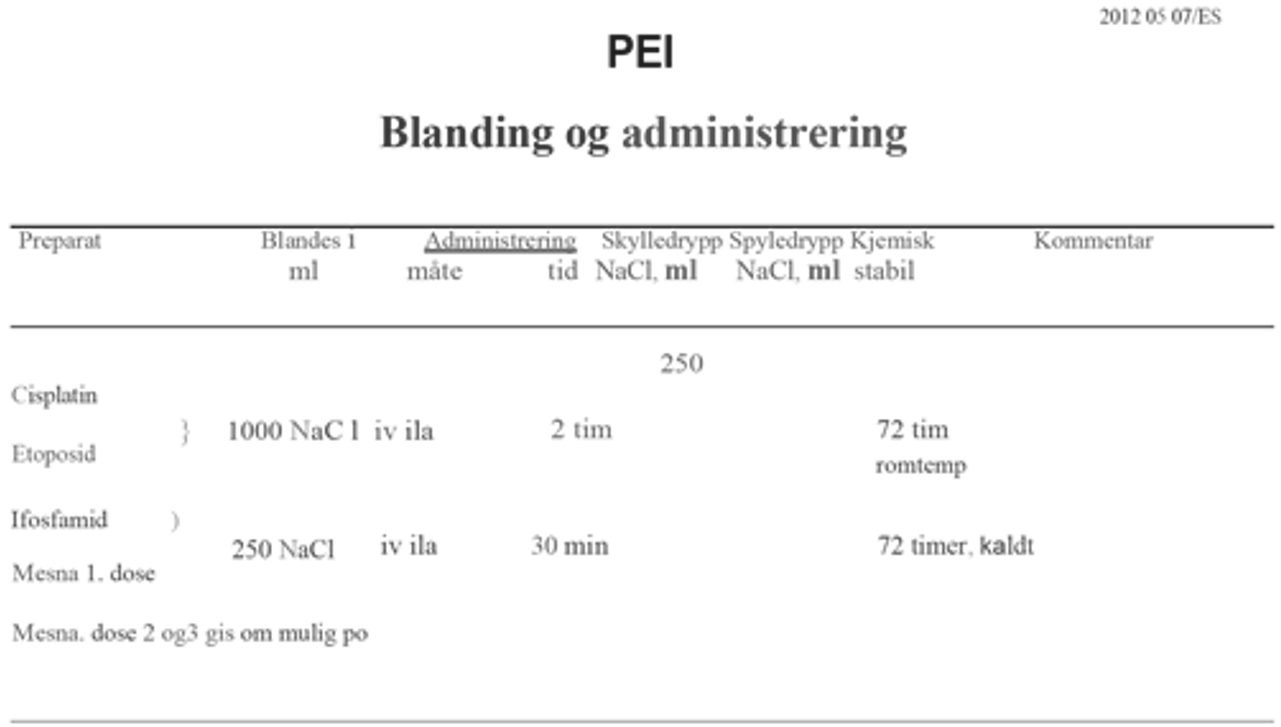
Prehydrering:
1000 NaClila 2 tim.
Hydrering under behandlingen:
I løpet av behandlingsdøgnet gis ytterligere minst 2000 ml væske po eller iv.
Posthydrering:
Døgnet etter siste cisplatininfusjon minst 2000 ml. Hvis pasienten ikke kan drikke denne mengden selv, skal væske gis iv.
Diuresen i løpet av behandlingsdøgnet samt døgnet etter den siste cisplatinbehandlingen skal være > 400 ml/4 tim. Måling starter samtidig med start av prehydrering.
XXV GOP
Sist faglig oppdatert: 15.04.2021
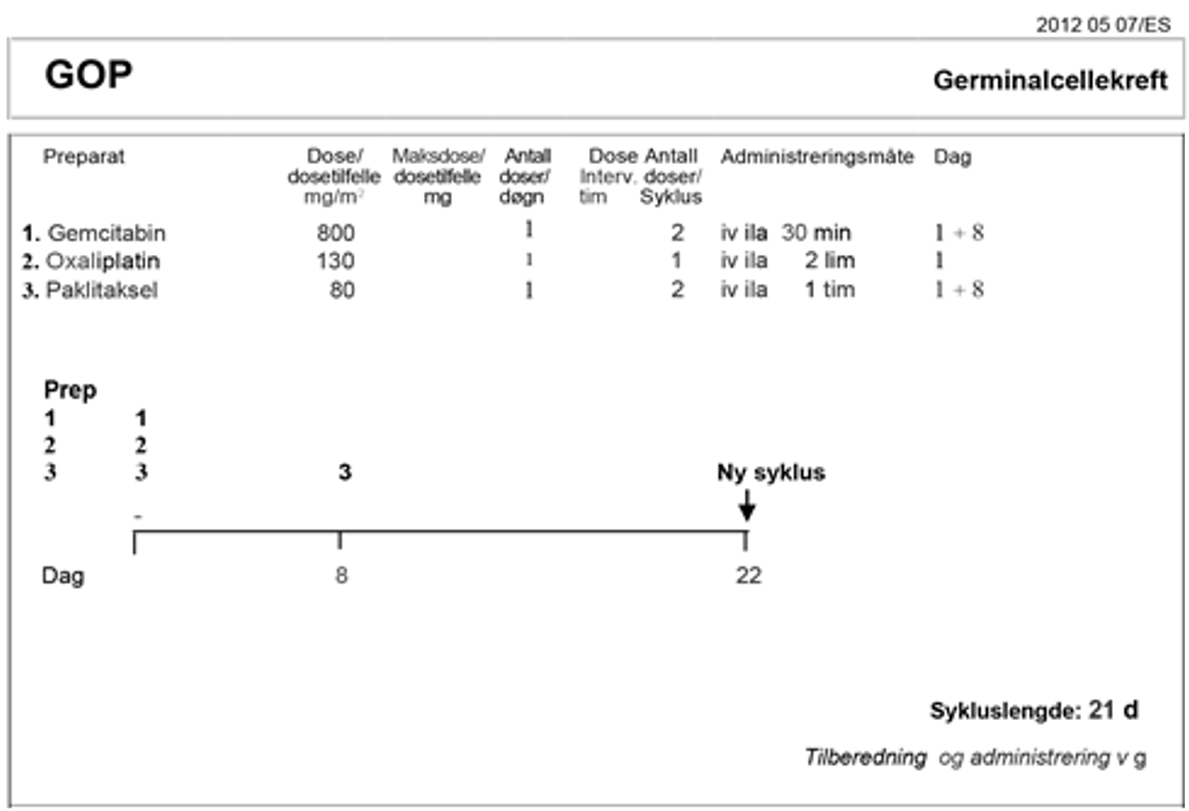
Spesielle tiltak
Oxaliplatin: Ved polynevropati gis substitusjon med kalsium og magnesium.
Paklitaksel premedisinering: 30 min før infusjon gis inj Betametason 6 mg iv. Gis kun dag 1 og 8 i syklus 1 hvis ingen uønskede reaksjoner har inntruffet. Inj. Clemastin 2 mg iv inj. Ranitidin 50 mg iv gis i samtlige sykluser.
Kontroll av puls og blodtrykk før og 15 min etter infusjonsstart dag 1 og 8 i syklus 1.
Akuttbrikke + PM for tiltak ved akutte allergiske reaksjoner skal være tilgjengelig. Lege skal kunne nås på personsøker. Se også "Håndtering av mild reaksjon ved Taxolinfusjon".
Anbefalinger for dosereduksjon


XXVI EMA-CO
Sist faglig oppdatert: 15.04.2021

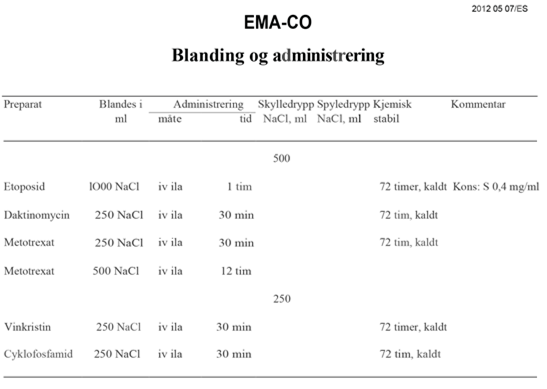
XXVII CE høydose kjemoterapi med autolog stamcellestøtte
Sist faglig oppdatert: 15.04.2021
|
Dag T –7 |
Innleggelse. Nylig evaluering av ukorrigert GFR ved bruk av Cr-EDTA/iohexol må ha blitt utført! Overveielser ifb. med nyrefunksjon1. Hvis kroppsoverflaten overstiger 2,2 m2, må det utføres individuell evaluering av pasientens konstitusjon. Start Allopurinol 300 mg x 1 Blodprøver inkludert AFP og ß-hCG |
|
Dag T –6 |
Antiemetisk profylakse Etoposid 560 mg/m2 i 4000 ml 0,9 % NaCl over 6 timer (maksimal konsentrasjon 0,4 mg/ml), maksimal dose 1340 mg2 Karboplatin 8 x (GFR + 25) mg i 1000 ml 5 % glukose over 1 time, GFR skal være den ukorrigerte/absolutte verdien |
|
Dag T –5 |
Antiemetisk profylakse Etoposid 560 mg/m2 i 4000 ml 0,9 % NaCl over 6 timer (maksimal konsentrasjon 0,4 mg/ml), maksimal dose 1340 mg2 Karboplatin 8 x (GFR + 25) mg i 1000 ml 5 % glukose over 1 time, maksimal dose 1085 mg3 GFR skal være den ukorrigerte/absolutte verdien |
|
Dag T –4 |
Antiemetisk profylakse Etoposid 560 mg/m2 i 4000 ml 0,9 % NaCl over 6 timer (maksimal konsentrasjon 0,4 mg/ml), maksimal dose 1340 mg2 Karboplatin 8 x (GFR + 25) mg i 1000 ml 5 % glukose over 1 time, GFR skal være den ukorrigerte/absolutte verdien |
|
Dag T –3 |
Antiemetisk profylakse Etoposid 560 mg/m2 i 4000 ml 0,9 % NaCl over 6 timer (maksimal konsentrasjon 0,4 mg/ml), maksimal dose 1340 mg2 Karboplatin 8 x (GFR + 25) mg i 1000 ml 5 % glukose over 1 time, GFR skal være den ukorrigerte/absolutte verdien |
|
Dag T –2 |
|
|
Dag T –1 |
Opphør av allopurinol |
|
Dag T –0 |
Autolog stamcelleinfusjon, ca. 72 timer etter avsluttet kjemoterapi |
|
Dag T +1 |
Start G-CSF 5μg/kg til nøytrofile er >1,0 i tre påfølgende dager |
|
1 Hvis ukorrigert/absolutt GFR >120 mL/min, eller pasientene har avvikende kroppssammensetning, for eksempel fedme eller ødem, kan følgende beregningsverktøy brukes www.egfr.se. Ved avvikende resultater mellom metoder kreves det ekstra årvåkenhet ved doseringen av karboplatin. Tilleggsmerknader: Pasienten bør overvåkes nøye med hensyn til væskebalanse. Pasienten bør motta minst 6 l væske/m2/24 timer. Hvis pasienter har væskeretensjon, bør det gis diuretika. Karboplatin kan også blandes i 0,9 % NaCl. Ved infeksjon under eller etter HDCT er aminoglykosider kontraindisert. |
|
XXVIII Diagram med halveringstid for ß-hCG
Sist faglig oppdatert: 15.04.2021
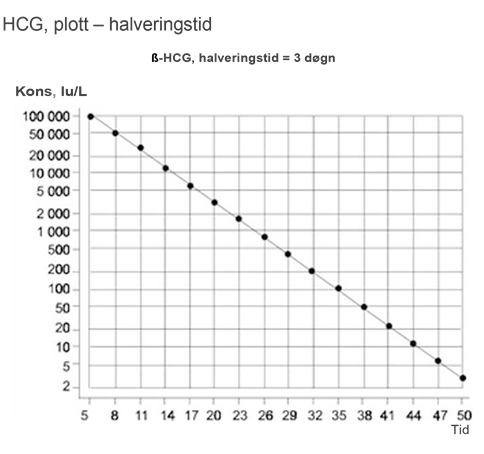
XXIX Diagram med halveringstid for AFP
Sist faglig oppdatert: 15.04.2021
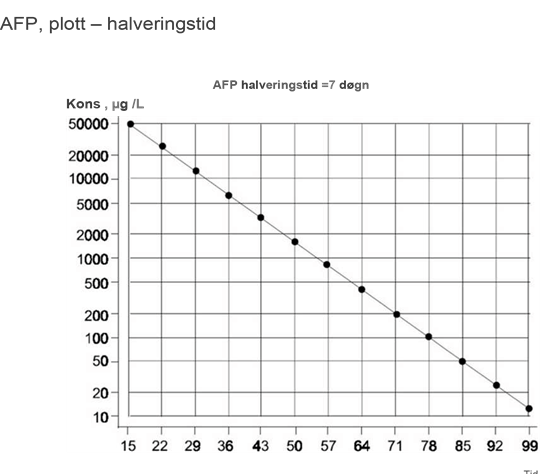
Referanser
Sist faglig oppdatert: 15.04.2021
Adams, S. C., DeLorey, D. S., Davenport, M. H., Fairey, A. S., North, S., & Courneya, K. S. (2018). Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br J Cancer, 118(10), 1313-1321. https://doi.org/10.1038/s41416-018-0044-7
Adra, N., Abonour, R., Althouse, S. K., Albany, C., Hanna, N. H., & Einhorn, L. H. (2017). High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. J Clin Oncol, 35(10), 1096-1102. https://doi.org/10.1200/JCO.2016.69.5395
Adra, N., Albany, C., Sonneburg, D., Tong, Y., Hanna, N., & Einhorn, L. (2014). A retrospective analysis of patients with poor-risk germ cell tumor (PRGCT) treated at Indiana University from 2000 to 2010. J Clin Oncol, 31, (suppl; abstra 4557.
Adra, N., Althouse, S. K., Liu, H., Brames, M. J., Hanna, N. H., Einhorn, L. H., & Albany, C. (2016). Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol, 27(5), 875-879. https://doi.org/10.1093/annonc/mdw045
Adra, N., Einhorn, L. H., Althouse, S. K., Ammakkanavar, N. R., Musapatika, D., Albany, C., . . . Hanna, N. H. (2018). Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol, 29(1), 209-214. https://doi.org/10.1093/annonc/mdx680
Al-Jebari, Y., Glimelius, I., Berglund Nord, C., Cohn-Cedermark, G., Stahl, O., Tandstad, T., . . . Giwercman, A. (2019). Cancer therapy and risk of congenital malformations in children fathered by men treated for testicular germ-cell cancer: A nationwide register study. PLoS Med, 16(6), e1002816. https://doi.org/10.1371/journal.pmed.1002816
Albany, C., & Einhorn, L. H. (2013). Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol, 25(3), 261-265. https://doi.org/10.1097/CCO.0b013e32835f085d
Albany, C., Kesler, K., & Cary, C. (2019). Management of Residual Mass in Germ Cell Tumors After Chemotherapy. Curr Oncol Rep, 21(1), 5. https://doi.org/10.1007/s11912-019-0758-6
Albers, P., Albrecht, W., Algaba, F., Bokemeyer, C., Cohn-Cedermark, G., Fizazi, K., . . . Laguna, M. P. (2011). EAU Guidelines on Testicular Cancer: 2011 Update. European Urology, 60(2), 304-319.
Albers, P., Bender, H., Yilmaz, H., Schoeneich, G., Biersack, H. J., & Mueller, S. C. (1999). Positron emission tomography in the clinical staging of patients with Stage I and II testicular germ cell tumors. Urology, 53(4), 808-811.
Albers, P., Goll, A., Bierhoff, E., Schoeneich, G., & Muller, S. C. (1999). Clinical course and histopathologic risk factor assessment in patients with bilateral testicular germ cell tumors. Urology, 54(4), 714-718.
Albers, P., Melchior, D., & Muller, S. C. (2003). Surgery in metastatic testicular cancer. Eur Urol, 44(2), 233-244. https://doi.org/10.1016/s0302-2838(03)00252-5
Albers, P., Siener, R., Krege, S., Schmelz, H.-U., Dieckmann, K.-P., Heidenreich, A., . . . Hartmann, M. (2008). Randomized Phase III Trial Comparing Retroperitoneal Lymph Node Dissection With One Course of Bleomycin and Etoposide Plus Cisplatin Chemotherapy in the Adjuvant Treatment of Clinical Stage I Nonseminomatous Testicular Germ Cell Tumors: AUO Trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol, JCO.2007.2012.0899. https://doi.org/10.1200/jco.2007.12.0899
Alexander, E. J., White, I. M., & Horwich, A. (2010). Update on management of seminoma. Indian J Urol, 26(1), 82-91. https://doi.org/10.4103/0970-1591.60451
Almstrup, K., Lobo, J., Morup, N., Belge, G., Rajpert-De Meyts, E., Looijenga, L. H. J., & Dieckmann, K. P. (2020). Application of miRNAs in the diagnosis and monitoring of testicular germ cell tumours. Nat Rev Urol, 17(4), 201-213. https://doi.org/10.1038/s41585-020-0296-x
Andreassen, K. E., Grotmol, T., Cvancarova, M. S., Johannesen, T. B., & Fossa, S. D. (2011). Risk of metachronous contralateral testicular germ cell tumors: a population-based study of 7,102 Norwegian patients (1953-2007). Int J Cancer, 129(12), 2867-2874. https://doi.org/10.1002/ijc.25943
Aparicio, J., Germa, J. R., Garcia del Muro, X., Maroto, P., Arranz, J. A., Saenz, A., . . . Sanchez, A. (2005). Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol, 23(34), 8717-8723.
https://pubmed.ncbi.nlm.nih.gov/16260698/
10.1200/JCO.2005.01.9810
Aparicio, J., Maroto, P., del Muro, X. G., Guma, J., Sanchez-Munoz, A., Margeli, M., . . . Germa, J. R. (2011). Risk-adapted treatment in clinical stage I testicular seminoma: the third Spanish Germ Cell Cancer Group study. J Clin Oncol, 29(35), 4677-4681. https://doi.org/10.1200/JCO.2011.36.0503
Bachner, M., Loriot, Y., Gross-Goupil, M., Zucali, P. A., Horwich, A., Germa-Lluch, J. R., . . . De Santis, M. (2011). 2-18fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann Oncol.
https://pubmed.ncbi.nlm.nih.gov/21460378/
10.1093/annonc/mdr052
Balawender, K., Orkisz, S., & Wisz, P. (2018). Testicular microlithiasis: what urologists should know. A review of the current literature. Cent European J Urol, 71(3), 310-314. https://doi.org/10.5173/ceju.2018.1728
Bamberg, M., Schmidberger, H., Meisner, C., Classen, J., Souchon, R., Weinknecht, S., . . . Flink, M. (1999). Radiotherapy for stages I and IIA/B testicular seminoma. Int J Cancer, 83(6), 823-827.
Bang, A. K., Petersen, J. H., Petersen, P. M., Andersson, A. M., Daugaard, G., & Jorgensen, N. (2009). Testosterone production is better preserved after 16 than 20 Gray irradiation treatment against testicular carcinoma in situ cells. Int J Radiat Oncol Biol Phys, 75(3), 672-676. https://doi.org/10.1016/j.ijrobp.2008.11.057
Baniel, J., Foster, R. S., Gonin, R., Messemer, J. E., Donohue, J. P., & Einhorn, L. H. (1995). Late relapse of testicular cancer. J Clin Oncol, 13(5), 1170-1176.
Baniel, J., Foster, R. S., Rowland, R. G., Bihrle, R., & Donohue, J. P. (1994). Complications of primary retroperitoneal lymph node dissection. J Urol, 152(2 Pt 1), 424-427.
Beck, S. D., Peterson, M. D., Bihrle, R., Donohue, J. P., & Foster, R. S. (2007). Short-term morbidity of primary retroperitoneal lymph node dissection in a contemporary group of patients. J Urol, 178(2), 504-506; discussion 506. https://doi.org/10.1016/j.juro.2007.03.123
Besse, B., Grunenwald, D., Flechon, A., Caty, A., Chevreau, C., Culine, S., . . . Fizazi, K. (2009). Nonseminomatous germ cell tumors: assessing the need for postchemotherapy contralateral pulmonary resection in patients with ipsilateral complete necrosis. J Thorac Cardiovasc Surg, 137(2), 448-452. https://doi.org/10.1016/j.jtcvs.2008.09.032
Beyer, J., Albers, P., Altena, R., Aparicio, J., Bokemeyer, C., Busch, J., . . . Wittekind, C. (2013). Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol, 24(4), 878-888. https://doi.org/10.1093/annonc/mds579
Beyer, J., Albers, P., Altena, R., Aparicio, J., Bokemeyer, C., Busch, J., . . . Wittekind, C. (2012). Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Annals of Oncology.
Bezan, A., Posch, F., Ploner, F., Bauernhofer, T., Pichler, M., Szkandera, J., . . . Stotz, M. (2017). Risk stratification for venous thromboembolism in patients with testicular germ cell tumors. PLoS One, 12(4), e0176283. https://doi.org/10.1371/journal.pone.0176283
Bhala, N., Coleman, J. M., Radstone, C. R., Horsman, J. M., George, J., Hancock, B. W., . . . Coleman, R. E. (2004). The management and survival of patients with advanced germ-cell tumours: improving outcome in intermediate and poor prognosis patients. Clin Oncol (R Coll Radiol), 16(1), 40-47. https://doi.org/10.1016/s0936-6555(03)00166-3
Bjerner, J., Biernat, D., Fossa, S. D., & Bjoro, T. (2009). Reference intervals for serum testosterone, SHBG, LH and FSH in males from the NORIP project. Scand J Clin Lab Invest, 69(8), 873-879 e871-811. https://doi.org/10.3109/00365510903380886
Bokemeyer, C., Hartmann, J. T., Fossa, S. D., Droz, J. P., Schmol, H. J., Horwich, A., . . . Einhorn, L. (2003). Extragonadal germ cell tumors: relation to testicular neoplasia and management options. APMIS, 111(1), 49-59; discussion 59-63. https://doi.org/10.1034/j.1600-0463.2003.11101081.x
Bokemeyer, C., Kollmannsberger, C., Stenning, S., Hartmann, J. T., Horwich, A., Clemm, C., . . . Oliver, T. (2004). Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: a pooled analysis of two randomised trials. Br J Cancer, 91(4), 683-687. https://doi.org/10.1038/sj.bjc.6602020
6602020 [pii]
Bokemeyer, C., Nichols, C. R., Droz, J. P., Schmoll, H. J., Horwich, A., Gerl, A., . . . Hartmann, J. T. (2002). Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol, 20(7), 1864-1873.
Bokemeyer, C., Oechsle, K., Honecker, F., Mayer, F., Hartmann, J. T., Waller, C. F., . . . Kollmannsberger, C. (2008). Combination chemotherapy with gemcitabine, oxaliplatin, and paclitaxel in patients with cisplatin-refractory or multiply relapsed germ-cell tumors: a study of the German Testicular Cancer Study Group. Ann Oncol, 19(3), 448-453.
https://pubmed.ncbi.nlm.nih.gov/18006893/
10.1093/annonc/mdm526
Bower, J. E., Bak, K., Berger, A., Breitbart, W., Escalante, C. P., Ganz, P. A., . . . American Society of Clinical, O. (2014). Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol, 32(17), 1840-1850. https://doi.org/10.1200/JCO.2013.53.4495
Brown, P. D., Pugh, S., Laack, N. N., Wefel, J. S., Khuntia, D., Meyers, C., . . . Radiation Therapy Oncology, G. (2013). Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol, 15(10), 1429-1437. https://doi.org/10.1093/neuonc/not114
Brydoy, M., Fossa, S. D., Klepp, O., Bremnes, R. M., Wist, E. A., Wentzel-Larsen, T., & Dahl, O. (2005). Paternity following treatment for testicular cancer. J Natl Cancer Inst, 97(21), 1580-1588. https://doi.org/10.1093/jnci/dji339
Brydoy, M., Oldenburg, J., Klepp, O., Bremnes, R. M., Wist, E. A., Wentzel-Larsen, T., . . . Fossa, S. D. (2009). Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst, 101(24), 1682-1695. https://doi.org/10.1093/jnci/djp413
Busch, J., Seidel, C., & Zengerling, F. (2016). Male Extragonadal Germ Cell Tumors of the Adult. Oncol Res Treat, 39(3), 140-144. https://doi.org/10.1159/000444271
Calaway, A. C., Tachibana, I., Masterson, T. A., Foster, R. S., Einhorn, L. H., & Cary, C. (2019). Oncologic Outcomes Following Surgical Management of Clinical Stage II Sex Cord Stromal Tumors. Urology, 127, 74-79. https://doi.org/10.1016/j.urology.2019.02.014
Capelouto, C. C., Clark, P. E., Ransil, B. J., & Loughlin, K. R. (1995). A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? J Urol, 153(3 Pt 2), 981-985.
Carver, B. S., Cronin, A. M., Eggener, S., Savage, C. J., Motzer, R. J., Bajorin, D., . . . Sheinfeld, J. (2010). The total number of retroperitoneal lymph nodes resected impacts clinical outcome after chemotherapy for metastatic testicular cancer. Urology, 75(6), 1431-1435. https://doi.org/10.1016/j.urology.2009.11.076
Cathomas, R., Klingbiel, D., Bernard, B., Lorch, A., Garcia Del Muro, X., Morelli, F., . . . Beyer, J. (2018). Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol, JCO1800210. https://doi.org/10.1200/JCO.18.00210
Cheney, S. M., Andrews, P. E., Leibovich, B. C., & Castle, E. P. (2015). Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int, 115(1), 114-120. https://doi.org/10.1111/bju.12804
Cho, J. S., Kaimakliotis, H. Z., Cary, C., Masterson, T. A., Beck, S., & Foster, R. (2017). Modified retroperitoneal lymph node dissection for post-chemotherapy residual tumour: a long-term update. BJU Int, 120(1), 104-108. https://doi.org/10.1111/bju.13844
Christensen, T. B., Engbaek, F., Marqversen, J., Nielsen, S. I., Kamby, C., & von der Maase, H. (1999). 125I-labelled human chorionic gonadotrophin (hCG) as an elimination marker in the evaluation of hCG decline during chemotherapy in patients with testicular cancer. Br J Cancer, 80(10), 1577-1581. https://doi.org/10.1038/sj.bjc.6690565
Christian, J. A., Huddart, R. A., Norman, A., Mason, M., Fossa, S., Aass, N., . . . Horwich, A. (2003). Intensive induction chemotherapy with CBOP/BEP in patients with poor prognosis germ cell tumors. J Clin Oncol, 21(5), 871-877. https://doi.org/10.1200/JCO.2003.05.155
Chung, P. W., Daugaard, G., Tyldesley, S., Panzarella, T., Kollmannsberger, C. K., Gospodarowicz, M., & Warde, P. (2010). Prognostic factors for relapse in stage I seminoma managed with surveillance: A validation study. J Clin Oncol, 28(7s), suppl; abstr 4535.
Chung, P. W., Gospodarowicz, M. K., Panzarella, T., Jewett, M. A., Sturgeon, J. F., Tew-George, B., . . . Warde, P. R. (2004). Stage II testicular seminoma: patterns of recurrence and outcome of treatment. Eur Urol, 45(6), 754-759; discussion 759-760. https://doi.org/10.1016/j.eururo.2004.01.020
Classen, J., Schmidberger, H., Meisner, C., Souchon, R., Sautter-Bihl, M. L., Sauer, R., . . . Bamberg, M. (2003). Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. J Clin Oncol, 21(6), 1101-1106.
Coogan, C. L., Foster, R. S., Simmons, G. R., Tognoni, P. G., Roth, B. J., & Donohue, J. P. (1998). Bilateral testicular tumors: management and outcome in 21 patients. Cancer, 83(3), 547-552.
Crona, D. J., Faso, A., Nishijima, T. F., McGraw, K. A., Galsky, M. D., & Milowsky, M. I. (2017). A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist, 22(5), 609-619. https://doi.org/10.1634/theoncologist.2016-0319
Culine, S., Kramar, A., Theodore, C., Geoffrois, L., Chevreau, C., Biron, P., . . . Genito-Urinary Group of the French Federation of Cancer Centers Trial, T. M. (2008). Randomized trial comparing bleomycin/etoposide/cisplatin with alternating cisplatin/cyclophosphamide/doxorubicin and vinblastine/bleomycin regimens of chemotherapy for patients with intermediate- and poor-risk metastatic nonseminomatous germ cell tumors: Genito-Urinary Group of the French Federation of Cancer Centers Trial T93MP. J Clin Oncol, 26(3), 421-427. https://doi.org/10.1200/JCO.2007.13.8461
Daneshmand, S., Albers, P., Fossa, S. D., Heidenreich, A., Kollmannsberger, C., Krege, S., . . . Wood, L. (2012). Contemporary management of postchemotherapy testis cancer. Eur Urol, 62(5), 867-876. https://doi.org/10.1016/j.eururo.2012.08.014
Daugaard, G., Petersen, P. M., & Rorth, M. (2003). Surveillance in stage I testicular cancer. APMIS, 111(1), 76-83; discussion 83-75. https://doi.org/10.1034/j.1600-0463.2003.11101111.x
Daugaard, G., Skoneczna, I., Aass, N., De Wit, R., De Santis, M., Dumez, H., . . . Schmoll, H. J. (2010). A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974). Ann Oncol.
https://pubmed.ncbi.nlm.nih.gov/21059637/
10.1093/annonc/mdq575
De Giorgi, U., Rosti, G., Aieta, M., Testore, F., Burattini, L., Fornarini, G., . . . Marangolo, M. (2006). Phase II study of oxaliplatin and gemcitabine salvage chemotherapy in patients with cisplatin-refractory nonseminomatous germ cell tumor. Eur Urol, 50(5), 1032-1038; discussion 1038-1039.
https://pubmed.ncbi.nlm.nih.gov/16757095/
10.1016/j.eururo.2006.05.011
De Giorgi, U., Rosti, G., Papiani, G., Aieta, M., Fochessati, F., Paoluzzi, L., . . . Marangolo, M. (2004). Weekly gemcitabine, paclitaxel, oxaliplatin combination chemotherapy in patients with Cisplatin-refractory germ cell tumor: preliminary experience. Am J Clin Oncol, 27(5), 457-460.
https://pubmed.ncbi.nlm.nih.gov/15596910/
De Santis, M., Becherer, A., Bokemeyer, C., Stoiber, F., Oechsle, K., Sellner, F., . . . Pont, J. (2004). 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol, 22(6), 1034-1039. https://doi.org/10.1200/JCO.2004.07.188
JCO.2004.07.188 [pii]
de Wit, M., Brenner, W., Hartmann, M., Kotzerke, J., Hellwig, D., Lehmann, J., . . . Bares, R. (2008). [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: results of the German multicentre trial. Ann Oncol, 19(9), 1619-1623. https://doi.org/10.1093/annonc/mdn170
de Wit, R., Roberts, J. T., Wilkinson, P. M., de Mulder, P. H., Mead, G. M., Fossa, S. D., . . . Collette, L. (2001). Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol, 19(6), 1629-1640.
de Wit, R., Skoneczna, I., Daugaard, G., De Santis, M., Garin, A., Aass, N., . . . Collette, L. (2012). Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983. J Clin Oncol, 30(8), 792-799. https://doi.org/10.1200/JCO.2011.37.0171
de Wit, R., Sleijfer, S., Kaye, S. B., Horwich, A., Mead, B., Sleijfer, D. T., & Stoter, G. (2007). Bleomycin and scuba diving: where is the harm? Lancet Oncol, 8(11), 954-955. https://doi.org/10.1016/S1470-2045(07)70321-2
Del Risco Kollerud, R., Blaasaas, K. G., Claussen, B., Nafstad, P., Cannon-Albright, L. A., Ruud, E., . . . Naess, O. (2018). Family history of cancer and the risk of childhood solid tumours: a Norwegian nationwide register-based cohort study. Br J Cancer, 118(6), 905-912. https://doi.org/10.1038/bjc.2017.493
DeSantis, M., Albrecht, W., Holtl, W., & Pont, J. (1999). Impact of cytotoxic treatment on long-term fertility in patients with germ-cell cancer. Int J Cancer, 83(6), 864-865.
Di Tonno, F., Tavolini, I. M., Belmonte, P., Bertoldin, R., Cossaro, E., Curti, P., . . . North-Eastern Uro-Oncological Group, I. (2009). Lessons from 52 patients with leydig cell tumor of the testis: the GUONE (North-Eastern Uro-Oncological Group, Italy) experience. Urologia Internationalis, 82(2), 152-157. https://doi.org/10.1159/000200790
Dieckmann, K. P., Albers, P., Classen, J., De Wit, M., Pichlmeier, U., Rick, O., . . . Kuczyk, M. (2005). Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol, 173(3), 824-829.https://pubmed.ncbi.nlm.nih.gov/15711278/
10.1097/01.ju.0000154013.96349.36
Dieckmann, K. P., Anheuser, P., Schmidt, S., Soyka-Hundt, B., Pichlmeier, U., Schriefer, P., . . . Ruf, C. G. (2015). Testicular prostheses in patients with testicular cancer - acceptance rate and patient satisfaction. BMC Urol, 15, 16. https://doi.org/10.1186/s12894-015-0010-0
Dieckmann, K. P., Bertolini, J., & Wulfing, C. (2019). Adult Granulosa Cell Tumor of the Testis: A Case Report with a Review of the Literature. Case Rep Urol, 2019, 7156154. https://doi.org/10.1155/2019/7156154
Dieckmann, K. P., Heinemann, V., Frey, U., Pichlmeier, U., & German Testicular Cancer Study, G. (2005). How harmful is contralateral testicular biopsy?--an analysis of serial imaging studies and a prospective evaluation of surgical complications. Eur Urol, 48(4), 662-672. https://doi.org/10.1016/j.eururo.2005.06.008
Dieckmann, K. P., Kulejewski, M., Pichlmeier, U., & Loy, V. (2007). Diagnosis of contralateral testicular intraepithelial neoplasia (TIN) in patients with testicular germ cell cancer: systematic two-site biopsies are more sensitive than a single random biopsy. Eur Urol, 51(1), 175-183; discussion 183-175.
https://pubmed.ncbi.nlm.nih.gov/16814456/
10.1016/j.eururo.2006.05.051
Domont, J., Massard, C., Patrikidou, A., Bossi, A., de Crevoisier, R., Rose, M., . . . Fizazi, K. (2013). A risk-adapted strategy of radiotherapy or cisplatin-based chemotherapy in stage II seminoma. Urol Oncol, 31(5), 697-705. https://doi.org/10.1016/j.urolonc.2011.04.004
Donat, S. M., & Levy, D. A. (1998). Bleomycin associated pulmonary toxicity: is perioperative oxygen restriction necessary? J Urol, 160(4), 1347-1352.
Donohue, J. P., & Foster, R. S. (1998). Retroperitoneal lymphadenectomy in staging and treatment. The development of nerve-sparing techniques. Urol Clin North Am, 25(3), 461-468. https://doi.org/10.1016/s0094-0143(05)70035-5
Doyle, D. M., & Einhorn, L. H. (2008). Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys, 70(5), 1361-1364. https://doi.org/10.1016/j.ijrobp.2007.11.005
Droz, J. P., Kramar, A., Biron, P., Pico, J. L., Kerbrat, P., Peny, J., . . . Culine, S. (2007). Failure of high-dose cyclophosphamide and etoposide combined with double-dose cisplatin and bone marrow support in patients with high-volume metastatic nonseminomatous germ-cell tumours: mature results of a randomised trial. Eur Urol, 51(3), 739-746; discussion 747-738.
https://pubmed.ncbi.nlm.nih.gov/17084512/
10.1016/j.eururo.2006.10.035
Eastham, J. A., Wilson, T. G., Russell, C., Ahlering, T. E., & Skinner, D. G. (1994). Surgical resection in patients with nonseminomatous germ cell tumor who fail to normalize serum tumor markers after chemotherapy. Urology, 43(1), 74-80. https://doi.org/10.1016/s0090-4295(94)80269-6
Eberhard, J., Stahl, O., Giwercman, Y., Cwikiel, M., Cavallin-Stahl, E., Lundin, K. B., . . . Giwercman, A. (2004). Impact of therapy and androgen receptor polymorphism on sperm concentration in men treated for testicular germ cell cancer: a longitudinal study. Hum. Reprod., 19(6), 1418-1425. https://doi.org/10.1093/humrep/deh231
Ehrlich, Y., Beck, S. D., Foster, R. S., Bihrle, R., & Einhorn, L. H. (2013). Serum tumor markers in testicular cancer. Urol Oncol, 31(1), 17-23. https://doi.org/10.1016/j.urolonc.2010.04.007
Ehrlich, Y., Brames, M. J., Beck, S. D., Foster, R. S., & Einhorn, L. H. (2010). Long-term follow-up of Cisplatin combination chemotherapy in patients with disseminated nonseminomatous germ cell tumors: is a postchemotherapy retroperitoneal lymph node dissection needed after complete remission? J Clin Oncol, 28(4), 531-536. https://doi.org/10.1200/JCO.2009.23.0714
Einhorn, L. H., Brames, M. J., Juliar, B., & Williams, S. D. (2007). Phase II study of paclitaxel plus gemcitabine salvage chemotherapy for germ cell tumors after progression following high-dose chemotherapy with tandem transplant. J Clin Oncol, 25(5), 513-516.
https://pubmed.ncbi.nlm.nih.gov/17290059/
10.1200/JCO.2006.07.7271
Einhorn, L. H., Williams, S. D., Chamness, A., Brames, M. J., Perkins, S. M., & Abonour, R. (2007). High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med, 357(4), 340-348.
https://pubmed.ncbi.nlm.nih.gov/17652649/
10.1056/NEJMoa067749
Fankhauser, C. D., Grogg, J. B., Hayoz, S., Wettstein, M. S., Dieckmann, K. P., Sulser, T., . . . Hermanns, T. (2019). Risk Factors and Treatment Outcomes of 1,375 Patients with Testicular Leydig Cell Tumors: Analysis of Published Case Series Data. J Urol, 101097JU0000000000000705. https://doi.org/10.1097/JU.0000000000000705
Farge, D., Frere, C., Connors, J. M., Ay, C., Khorana, A. A., Munoz, A., . . . Cancer advisory, p. (2019). 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol, 20(10), e566-e581. https://doi.org/10.1016/S1470-2045(19)30336-5
Feldman, D. R., Lorch, A., Kramar, A., Albany, C., Einhorn, L. H., Giannatempo, P., . . . Powles, T. (2016). Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options--An Analysis From the Global Germ Cell Cancer Group. J Clin Oncol, 34(4), 345-351. https://doi.org/10.1200/JCO.2015.62.7000
Feldman, D. R., Sheinfeld, J., Bajorin, D. F., Fischer, P., Turkula, S., Ishill, N., . . . Motzer, R. J. (2010). TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol, 28(10), 1706-1713. https://doi.org/10.1200/JCO.2009.25.1561
Fizazi, K., Delva, R., Caty, A., Chevreau, C., Kerbrat, P., Rolland, F., . . . Laplanche, A. (2014). A risk-adapted study of cisplatin and etoposide, with or without ifosfamide, in patients with metastatic seminoma: results of the GETUG S99 multicenter prospective study. Eur Urol, 65(2), 381-386. https://doi.org/10.1016/j.eururo.2013.09.004
Fizazi, K., Oldenburg, J., Dunant, A., Chen, I., Salvioni, R., Hartmann, J. T., . . . Fromont, G. (2008). Assessing prognosis and optimizing treatment in patients with postchemotherapy viable nonseminomatous germ-cell tumors (NSGCT): results of the sCR2 international study. Annals of Oncology, 19(2), 259-264.
https://pubmed.ncbi.nlm.nih.gov/20550599/
Fizazi, K., Pagliaro, L., Laplanche, A., Flechon, A., Mardiak, J., Geoffrois, L., . . . Culine, S. (2014). Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol, 15(13), 1442-1450. https://doi.org/10.1016/S1470-2045(14)70490-5
Fizazi, K., Prow, D. M., Do, K. A., Wang, X., Finn, L., Kim, J., . . . Amato, R. J. (2002). Alternating dose-dense chemotherapy in patients with high volume disseminated non-seminomatous germ cell tumours. Br J Cancer, 86(10), 1555-1560. https://doi.org/10.1038/sj.bjc.6600272
Fizazi, K., Tjulandin, S., Salvioni, R., Germa-Lluch, J. R., Bouzy, J., Ragan, D., . . . Mahe, C. (2001). Viable malignant cells after primary chemotherapy for disseminated nonseminomatous germ cell tumors: prognostic factors and role of postsurgery chemotherapy--results from an international study group. J Clin Oncol, 19(10), 2647-2657. https://doi.org/10.1200/JCO.2001.19.10.2647
Flechon, A., Bompas, E., Biron, P., & Droz, J. P. (2002). Management of post-chemotherapy residual masses in advanced seminoma. J Urol, 168(5), 1975-1979.
https://pubmed.ncbi.nlm.nih.gov/12394688/
Forsberg, L., Dale, L., Hoiem, L., Magnusson, A., Mikulowski, P., Olsson, A. M., . . . Stenwig, A. E. (1986). Computed tomography in early stages of testicular carcinoma. Size of normal retroperitoneal lymph nodes and lymph nodes in patients with metastases in stage II A. A SWENOTECA study: Swedish-Norwegian Testicular Cancer Project. Acta Radiol Diagn (Stockh), 27(5), 569-574. https://doi.org/10.1177/028418518602700516
Forskrift om prioritering av helsetjenester, rett til nødvendig helsehjelp fra spesialisthelsetjenesten, rett til behandling i utlandet og om klagenemnd (prioriteringsforskriften). FOR-2000-12-01-1208. nr. 1208.
Fossa, S. D., Bokemeyer, C., Gerl, A., Culine, S., Jones, W. G., Mead, G. M., . . . Tjulandin, S. (1999). Treatment outcome of patients with brain metastases from malignant germ cell tumors. Cancer, 85(4), 988-997.
https://pubmed.ncbi.nlm.nih.gov/10091779/
Fossa, S. D., Gilbert, E., Dores, G. M., Chen, J., McGlynn, K. A., Schonfeld, S., . . . Travis, L. B. (2007). Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst, 99(7), 533-544.
https://pubmed.ncbi.nlm.nih.gov/17405998/
10.1093/jnci/djk111
Fossa, S. D., Kaye, S. B., Mead, G. M., Cullen, M., de Wit, R., Bodrogi, I., . . . Collette, L. (1998). Filgrastim during combination chemotherapy of patients with poor-prognosis metastatic germ cell malignancy. European Organization for Research and Treatment of Cancer, Genito-Urinary Group, and the Medical Research Council Testicular Cancer Working Party, Cambridge, United Kingdom. J Clin Oncol, 16(2), 716-724. https://doi.org/10.1200/JCO.1998.16.2.716
Fossa, S. D., Ous, S., Lien, H. H., & Stenwig, A. E. (1989). Post-chemotherapy lymph node histology in radiologically normal patients with metastatic nonseminomatous testicular cancer. J Urol, 141(3), 557-559. https://doi.org/10.1016/s0022-5347(17)40892-5
Fossa, S. D., Paluchowska, B., Horwich, A., Kaiser, G., de Mulder, P. H., Koriakine, O., . . . Group, E. G. (2005). Intensive induction chemotherapy with C-BOP/BEP for intermediate- and poor-risk metastatic germ cell tumours (EORTC trial 30948). Br J Cancer, 93(11), 1209-1214. https://doi.org/10.1038/sj.bjc.6602830
Fossa, S. D., Stenning, S. P., Gerl, A., Horwich, A., Clark, P. I., Wilkinson, P. M., . . . Cook, P. A. (1999). Prognostic factors in patients progressing after cisplatin-based chemotherapy for malignant non-seminomatous germ cell tumours. Br J Cancer, 80(9), 1392-1399. https://doi.org/10.1038/sj.bjc.6690534
Fossa, S. D., Aass, N., Harvei, S., & Tretli, S. (2004). Increased mortality rates in young and middle-aged patients with malignant germ cell tumours. Br J Cancer, 90(3), 607-612. https://doi.org/10.1038/sj.bjc.6601558
Fossa, S. D., Aass, N., Winderen, M., Bormer, O. P., & Olsen, D. R. (2002). Long-term renal function after treatment for malignant germ-cell tumours. Ann Oncol, 13(2), 222-228.
Foster, R. S., Baniel, J., Leibovitch, I., Curran, M., Bihrle, R., Rowland, R., & Donohue, J. P. (1996). Teratoma in the orchiectomy specimen and volume of metastasis are predictors of retroperitoneal teratoma in low stage nonseminomatous testis cancer. J Urol, 155(6), 1943-1945.
Fox, E. P., Weathers, T. D., Williams, S. D., Loehrer, P. J., Ulbright, T. M., Donohue, J. P., & Einhorn, L. H. (1993). Outcome analysis for patients with persistent nonteratomatous germ cell tumor in postchemotherapy retroperitoneal lymph node dissections. J Clin Oncol, 11(7), 1294-1299. https://doi.org/10.1200/JCO.1993.11.7.1294
Frisina, R. D., Wheeler, H. E., Fossa, S. D., Kerns, S. L., Fung, C., Sesso, H. D., . . . Travis, L. B. (2016). Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol, 34(23), 2712-2720. https://doi.org/10.1200/JCO.2016.66.8822
Fung, C., Fossa, S. D., Milano, M. T., Oldenburg, J., & Travis, L. B. (2013). Solid tumors after chemotherapy or surgery for testicular nonseminoma: a population-based study. J Clin Oncol, 31(30), 3807-3814. https://doi.org/10.1200/JCO.2013.50.3409
Gennaro, N., Sconfienza, L. M., Ambrogi, F., Boveri, S., & Lanza, E. (2019). Thermal ablation to relieve pain from metastatic bone disease: a systematic review. Skeletal Radiol, 48(8), 1161-1169. https://doi.org/10.1007/s00256-018-3140-0
George, D. W., Foster, R. S., Hromas, R. A., Robertson, K. A., Vance, G. H., Ulbright, T. M., . . . Einhorn, L. H. (2003). Update on late relapse of germ cell tumor: a clinical and molecular analysis. J Clin Oncol, 21(1), 113-122. https://doi.org/10.1200/JCO.2003.03.019
Gerdtsson, A., Hakansson, U., Tornblom, M., Jancke, G., Negaard, H. F. S., Glimelius, I., . . . Kjellman, A. (2019). Surgical Complications in Postchemotherapy Retroperitoneal Lymph Node Dissection for Nonseminoma Germ Cell Tumour: A Population-based Study from the Swedish Norwegian Testicular Cancer Group. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2019.08.002
Gerl, A., Clemm, C., Schmeller, N., Hentrich, M., Lamerz, R., & Wilmanns, W. (1997). Late relapse of germ cell tumors after cisplatin-based chemotherapy. Ann Oncol, 8(1), 41-47. https://doi.org/10.1023/a:1008253323854
Germa-Lluch, J. R., Garcia del Muro, X., Tabernero, J. M., Sanchez, M., Aparicio, J., Alba, E., & Barnadas, A. (1999). BOMP/EPI intensive alternating chemotherapy for IGCCC poor-prognosis germ-cell tumors: the Spanish Germ-Cell Cancer Group experience (GG). Ann Oncol, 10(3), 289-293. https://doi.org/10.1023/a:1008351022211
Ghezzi, M., Berretta, M., Bottacin, A., Palego, P., Sartini, B., Cosci, I., . . . Garolla, A. (2016). Impact of Bep or Carboplatin Chemotherapy on Testicular Function and Sperm Nucleus of Subjects with Testicular Germ Cell Tumor. Front Pharmacol, 7, 122. https://doi.org/10.3389/fphar.2016.00122
Ghodoussipour, S., & Daneshmand, S. (2020). Surgical strategies for postchemotherapy testis cancer. Transl Androl Urol, 9(Suppl 1), S74-S82. https://doi.org/10.21037/tau.2019.09.43
Giannatempo, P., Pond, G. R., Sonpavde, G., Albany, C., Loriot, Y., Sweeney, C. J., . . . Cary, C. (2016). Treatment and Clinical Outcomes of Patients with Teratoma with Somatic-Type Malignant Transformation: An International Collaboration. J Urol, 196(1), 95-100. https://doi.org/10.1016/j.juro.2015.12.082
Gizzi, M., Oberic, L., Massard, C., Poterie, A., Le Teuff, G., Loriot, Y., . . . Fizazi, K. (2016). Predicting and preventing thromboembolic events in patients receiving cisplatin-based chemotherapy for germ cell tumours. Eur J Cancer, 69, 151-157. https://doi.org/10.1016/j.ejca.2016.10.003
Griggs, J. J., Mangu, P. B., Anderson, H., Balaban, E. P., Dignam, J. J., Hryniuk, W. M., . . . American Society of Clinical, O. (2012). Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol, 30(13), 1553-1561. https://doi.org/10.1200/JCO.2011.39.9436
Grogg, J., Schneider, K., Bode, P. K., Kranzbuhler, B., Eberli, D., Sulser, T., . . . Fankhauser, C. D. (2020). Sertoli Cell Tumors of the Testes: Systematic Literature Review and Meta-Analysis of Outcomes in 435 Patients. Oncologist. https://doi.org/10.1634/theoncologist.2019-0692
Groot, H. J., Lubberts, S., de Wit, R., Witjes, J. A., Kerst, J. M., de Jong, I. J., . . . Schaapveld, M. (2018). Risk of Solid Cancer After Treatment of Testicular Germ Cell Cancer in the Platinum Era. J Clin Oncol, 36(24), 2504-2513. https://doi.org/10.1200/JCO.2017.77.4174
Gupta, S. K., Lindemulder, E. A., & Sathyan, G. (2000). Modeling of circadian testosterone in healthy men and hypogonadal men. J Clin Pharmacol, 40(7), 731-738. https://doi.org/10.1177/00912700022009486
Hale, G. R., Teplitsky, S., Truong, H., Gold, S. A., Bloom, J. B., & Agarwal, P. K. (2018). Lymph node imaging in testicular cancer. Transl Androl Urol, 7(5), 864-874. https://doi.org/10.21037/tau.2018.07.18
Hall, E. J., & Wuu, C. S. (2003). Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys, 56(1), 83-88.
Hamid, A. A., Markt, S. C., Vicier, C., McDermott, K., Richardson, P., Ho, V. T., & Sweeney, C. J. (2019). Autologous Stem-Cell Transplantation Outcomes for Relapsed Metastatic Germ-Cell Tumors in the Modern Era. Clin Genitourin Cancer, 17(1), 58-64 e51. https://doi.org/10.1016/j.clgc.2018.09.009
Handbook for reporting results of cancer treatment. (1979). Geneva, Switzerland
Harland, S. J., Cook, P. A., Fossa, S. D., Horwich, A., Mead, G. M., Parkinson, M. C., . . . Stenning, S. P. (1998). Intratubular germ cell neoplasia of the contralateral testis in testicular cancer: defining a high risk group. J Urol, 160(4), 1353-1357.
Hartmann, J. T., Candelaria, M., Kuczyk, M. A., Schmoll, H. J., & Bokemeyer, C. (1997). Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer, 33(6), 843-847.
Hartmann, J. T., Fossa, S. D., Nichols, C. R., Droz, J. P., Horwich, A., Gerl, A., . . . Bokemeyer, C. (2001). Incidence of metachronous testicular cancer in patients with extragonadal germ cell tumors. J Natl Cancer Inst, 93(22), 1733-1738. https://doi.org/10.1093/jnci/93.22.1733
Hartmann, J. T., Gauler, T., Metzner, B., Gerl, A., Casper, J., Rick, O., . . . German Testicular Cancer Study, G. (2007). Phase I/II study of sequential dose-intensified ifosfamide, cisplatin, and etoposide plus paclitaxel as induction chemotherapy for poor prognosis germ cell tumors by the German Testicular Cancer Study Group. J Clin Oncol, 25(36), 5742-5747. https://doi.org/10.1200/JCO.2007.11.9099
Hartmann, J. T., & Lipp, H. P. (2006). Camptothecin and podophyllotoxin derivatives: inhibitors of topoisomerase I and II - mechanisms of action, pharmacokinetics and toxicity profile. Drug Saf, 29(3), 209-230. https://doi.org/10.2165/00002018-200629030-00005
Hartmann, J. T., Nichols, C. R., Droz, J. P., Horwich, A., Gerl, A., Fossa, S. D., . . . Bokemeyer, C. (2000). Hematologic disorders associated with primary mediastinal nonseminomatous germ cell tumors. J Natl Cancer Inst, 92(1), 54-61.
Hashimoto, K., Fujimoto, H., Kouno, T., Koseki, M., Yonemori, K., Hirata, T., . . . Fujiwara, Y. (2012). The incidence and management of metachronous testicular germ cell tumors in patients with extragonadal germ cell tumors. Urol Oncol, 30(3), 319-324. https://doi.org/10.1016/j.urolonc.2010.02.008
Haugnes, H. S., Laurell, A., Stierner, U., Bremnes, R. M., Dahl, O., Cavallin-Stahl, E., & Cohn-Cedermark, G. (2012). High-dose chemotherapy with autologous stem cell support in patients with metastatic non-seminomatous testicular cancer - a report from the Swedish Norwegian Testicular Cancer Group (SWENOTECA). Acta Oncol, 51(2), 168-176. https://doi.org/10.3109/0284186X.2011.641507
Haugnes, H. S., Oldenburg, J., & Bremnes, R. M. (2015). Pulmonary and cardiovascular toxicity in long-term testicular cancer survivors. Urol Oncol, 33(9), 399-406. https://doi.org/10.1016/j.urolonc.2014.11.012
Haugnes, H. S., Stenklev, N. C., Brydoy, M., Dahl, O., Wilsgaard, T., Laukli, E., & Fossa, S. D. (2018). Hearing loss before and after cisplatin-based chemotherapy in testicular cancer survivors: a longitudinal study. Acta Oncol, 57(8), 1075-1083. https://doi.org/10.1080/0284186X.2018.1433323
Haugnes, H. S., Wethal, T., Aass, N., Dahl, O., Klepp, O., Langberg, C. W., . . . Fossa, S. D. (2010). Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol, 28(30), 4649-4657.
https://pubmed.ncbi.nlm.nih.gov/20855830/
10.1200/JCO.2010.29.9362
Haugnes, H. S., Aass, N., Fossa, S. D., Dahl, O., Brydoy, M., Aasebo, U., . . . Bremnes, R. M. (2009). Pulmonary function in long-term survivors of testicular cancer. J Clin Oncol, 27(17), 2779-2786. https://doi.org/10.1200/JCO.2008.18.5181
Haugnes, H. S., Aass, N., Fossa, S. D., Dahl, O., Klepp, O., Wist, E. A., . . . Bremnes, R. M. (2007). Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol, 18(2), 241-248.
https://pubmed.ncbi.nlm.nih.gov/17060482/
10.1093/annonc/mdl372
Heidenreich, A., Albers, P., Hartmann, M., Kliesch, S., Kohrmann, K. U., Krege, S., . . . German Testicular Cancer Study, G. (2003). Complications of primary nerve sparing retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell tumors of the testis: experience of the German Testicular Cancer Study Group. J Urol, 169(5), 1710-1714. https://doi.org/10.1097/01.ju.0000060960.18092.54
Heidenreich, A., Paffenholz, P., Nestler, T., & Pfister, D. (2018). Primary and Postchemotherapy Retroperitoneal Lymphadenectomy for Testicular Cancer. Oncol Res Treat, 41(6), 370-378. https://doi.org/10.1159/000489508
Heidenreich, A., Paffenholz, P., Nestler, T., Pfister, D., & Daneshmand, S. (2020). Role of primary retroperitoneal lymph node dissection in stage I and low-volume metastatic germ cell tumors. Curr Opin Urol, 30(2), 251-257. https://doi.org/10.1097/MOU.0000000000000736
Heidenreich, A., Pfister, D., Witthuhn, R., Thuer, D., & Albers, P. (2009). Postchemotherapy retroperitoneal lymph node dissection in advanced testicular cancer: radical or modified template resection. Eur Urol, 55(1), 217-224. https://doi.org/10.1016/j.eururo.2008.09.027
Hellesnes, R., Kvammen, O., Myklebust, T. A., Bremnes, R. M., Karlsdottir, A., Negaard, H. F. S., . . . Haugnes, H. S. (2019). Continuing increased risk of second cancer in long-term testicular cancer survivors after treatment in the cisplatin era. Int J Cancer. https://doi.org/10.1002/ijc.32704
Herr, H. W., Sheinfeld, J., Puc, H. S., Heelan, R., Bajorin, D. F., Mencel, P., . . . Motzer, R. J. (1997a). Surgery for a post-chemotherapy residual mass in seminoma. J Urol, 157(3), 860-862.
Herr, H. W., Sheinfeld, J., Puc, H. S., Heelan, R., Bajorin, D. F., Mencel, P., . . . Motzer, R. J. (1997b). Surgery for a post-chemotherapy residual mass in seminoma. J Urol, 157(3), 860-862.
https://pubmed.ncbi.nlm.nih.gov/9072586/
Hiester, A., Nini, A., Fingerhut, A., Grosse Siemer, R., Winter, C., Albers, P., & Lusch, A. (2018). Preservation of Ejaculatory Function After Postchemotherapy Retroperitoneal Lymph Node Dissection (PC-RPLND) in Patients With Testicular Cancer: Template vs. Bilateral Resection. Front Surg, 5, 80. https://doi.org/10.3389/fsurg.2018.00080
Hinton, S., Catalano, P. J., Einhorn, L. H., Nichols, C. R., David Crawford, E., Vogelzang, N., . . . Loehrer, P. J., Sr. (2003). Cisplatin, etoposide and either bleomycin or ifosfamide in the treatment of disseminated germ cell tumors: final analysis of an intergroup trial. Cancer, 97(8), 1869-1875. https://doi.org/10.1002/cncr.11271
Hinze, A. M., & Wigley, F. M. (2018). Pharmacotherapy Options in the Management of Raynaud's Phenomenon. Curr Treatm Opt Rheumatol, 4(3), 235-254. https://doi.org/10.1007/s40674-018-0102-6
Hoenemeyer, T. W., Kaptchuk, T. J., Mehta, T. S., & Fontaine, K. R. (2018). Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial. Sci Rep, 8(1), 2784. https://doi.org/10.1038/s41598-018-20993-y
Hofmockel, G., Gruss, A., & Theiss, M. (1996). Chemotherapy in advanced seminoma and the role of postcytostatic retroperitoneal lymph node dissection. Urologia Internationalis, 57(1), 38-42. https://doi.org/10.1159/000282874
Honecker, F., Aparicio, J., Berney, D., Beyer, J., Bokemeyer, C., Cathomas, R., . . . Horwich, A. (2018). ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol, 29(8), 1658-1686. https://doi.org/10.1093/annonc/mdy217
Horwich, A., Fossa, S. D., Stenning, S., Bliss, J., & Hall, E. J. (2010). Risk of second cancers among a cohort of 2,703 long-term survivors of testicular seminoma treated with radiotherapy. J Clin Oncol, 28(7s), suppl; abstr 4538.
Horwich, A., Oliver, R. T., Wilkinson, P. M., Mead, G. M., Harland, S. J., Cullen, M. H., . . . Stenning, S. P. (2000). A medical research council randomized trial of single agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. MRC Testicular Tumour Working Party. Br J Cancer, 83(12), 1623-1629. https://doi.org/10.1054/bjoc.2000.1498
S0007092000914988 [pii]
Houck, W., Abonour, R., Vance, G., & Einhorn, L. H. (2004). Secondary leukemias in refractory germ cell tumor patients undergoing autologous stem-cell transplantation using high-dose etoposide. J Clin Oncol, 22(11), 2155-2158. https://doi.org/10.1200/JCO.2004.11.054
Hu, B., & Daneshmand, S. (2018). Retroperitoneal Lymph Node Dissection as Primary Treatment for Metastatic Seminoma. Adv Urol, 2018, 7978958. https://doi.org/10.1155/2018/7978958
Hu, B., Shah, S., Shojaei, S., & Daneshmand, S. (2015). Retroperitoneal Lymph Node Dissection as First-Line Treatment of Node-Positive Seminoma. Clin Genitourin Cancer, 13(4), e265-e269. https://doi.org/10.1016/j.clgc.2015.01.002
Hu, R., Ulbright, T. M., & Young, R. H. (2019). Spermatocytic Seminoma: A Report of 85 Cases Emphasizing Its Morphologic Spectrum Including Some Aspects Not Widely Known. Am J Surg Pathol, 43(1), 1-11. https://doi.org/10.1097/PAS.0000000000001001
Huddart, R. A., Gabe, R., Cafferty, F. H., Pollock, P., White, J. D., Shamash, J., . . . National Cancer Research Institute Testis Cancer Clinical Studies, G. (2015). A randomised phase 2 trial of intensive induction chemotherapy (CBOP/BEP) and standard BEP in poor-prognosis germ cell tumours (MRC TE23, CRUK 05/014, ISRCTN 53643604). Eur Urol, 67(3), 534-543. https://doi.org/10.1016/j.eururo.2014.06.034
Huddart, R. A., Norman, A., Shahidi, M., Horwich, A., Coward, D., Nicholls, J., & Dearnaley, D. P. (2003). Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol, 21(8), 1513-1523. https://doi.org/10.1200/JCO.2003.04.173
JCO.2003.04.173 [pii]
Huddart, R. A., O'Doherty, M. J., Padhani, A., Rustin, G. J., Mead, G. M., Joffe, J. K., . . . Group, N. T. T. C. S. (2007). 18fluorodeoxyglucose positron emission tomography in the prediction of relapse in patients with high-risk, clinical stage I nonseminomatous germ cell tumors: preliminary report of MRC Trial TE22--the NCRI Testis Tumour Clinical Study Group. J Clin Oncol, 25(21), 3090-3095. https://doi.org/10.1200/JCO.2006.09.3831
ICRU, I. C. o. R. U. a. M. (1993). Prescribing, recording and reporting photon beam therapy report 50. Oxford University Press, Oxford, United Kingdom.
ICRU, I. C. o. R. U. a. M. (2010). Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). ICRU Report 83. J ICRU.
International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. (1997). J Clin Oncol, 15(2), 594-603.
Iwamoto, H., Izumi, K., Natsagdorj, A., Makino, T., Nohara, T., Shigehara, K., . . . Mizokami, A. (2018). Effectiveness and Safety of Pegfilgrastim in BEP Treatment for Patients with Germ Cell Tumor. In Vivo, 32(4), 899-903. https://doi.org/10.21873/invivo.11326
Jacobsen, K. D., Ous, S., Waehre, H., Trasti, H., Stenwig, A. E., Lien, H. H., . . . Fossa, S. D. (1999). Ejaculation in testicular cancer patients after post-chemotherapy retroperitoneal lymph node dissection. Br J Cancer, 80(1-2), 249-255. https://doi.org/10.1038/sj.bjc.6690347
Jacus, M. O., Daryani, V. M., Harstead, K. E., Patel, Y. T., Throm, S. L., & Stewart, C. F. (2016). Pharmacokinetic Properties of Anticancer Agents for the Treatment of Central Nervous System Tumors: Update of the Literature. Clin Pharmacokinet, 55(3), 297-311. https://doi.org/10.1007/s40262-015-0319-6
Joel, S. P., Shah, R., Clark, P. I., & Slevin, M. L. (1996). Predicting etoposide toxicity: relationship to organ function and protein binding. J Clin Oncol, 14(1), 257-267. https://doi.org/10.1200/JCO.1996.14.1.257
Jordan, B., Margulies, A., Cardoso, F., Cavaletti, G., Haugnes, H. S., Jahn, P., . . . office@eano.eu, E. G. C. E. a. (2020). Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol, 31(10), 1306-1319. https://doi.org/10.1016/j.annonc.2020.07.003
Kamischke, A., & Nieschlag, E. (2002). Update on medical treatment of ejaculatory disorders. Int J Androl, 25(6), 333-344. https://doi.org/10.1046/j.1365-2605.2002.00379.x
Kesler, K. A., Rieger, K. M., Hammoud, Z. T., Kruter, L. E., Perkins, S. M., Turrentine, M. W., . . . Brown, J. W. (2008). A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg, 85(2), 371-378. https://doi.org/10.1016/j.athoracsur.2007.09.020
Key, N. S., Khorana, A. A., Kuderer, N. M., Bohlke, K., Lee, A. Y. Y., Arcelus, J. I., . . . Falanga, A. (2020). Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol, 38(5), 496-520. https://doi.org/10.1200/JCO.19.01461
Khetpal, R., Katz, M. D., Cox, M., & Arnaoutakis, K. (2014). The role of salvage hemiscrotectomy in testicular cancer after scrotal contamination: a case report and literature review. Clin Genitourin Cancer, 12(3), e103-105. https://doi.org/10.1016/j.clgc.2013.12.004
Kier, M. G., Hansen, M. K., Lauritsen, J., Mortensen, M. S., Bandak, M., Agerbaek, M., . . . Daugaard, G. (2016). Second Malignant Neoplasms and Cause of Death in Patients With Germ Cell Cancer: A Danish Nationwide Cohort Study. JAMA Oncol, 2(12), 1624-1627. https://doi.org/10.1001/jamaoncol.2016.3651
Kleinschmidt, K., Dieckmann, K. P., Georgiew, A., Loy, V., & Weissbach, L. (2009). Chemotherapy is of limited efficacy in the control of contralateral testicular intraepithelial neoplasia in patients with testicular germ cell cancer. Oncology, 77(1), 33-39. https://doi.org/10.1159/000218202
Klepp, O., Olsson, A. M., Ous, S., Nilsson, S., Hoisaether, P. A., & Tveter, K. (1991). Early clinical stages of nonseminomatous testis cancer. Evaluation of the primary treatment and follow-up procedures of the SWENOTECA project. Scand J Urol Nephrol, 25(3), 179-190.
Kliesch, S., Thomaidis, T., Schutte, B., Puhse, G., Kater, B., Roth, S., & Bergmann, M. (2003). Update on the diagnostic safety for detection of testicular intraepithelial neoplasia (TIN). APMIS, 111(1), 70-74; discussion 75.
Kollmannsberger, C., Beyer, J., Liersch, R., Schoeffski, P., Metzner, B., Hartmann, J. T., . . . Bokemeyer, C. (2004). Combination chemotherapy with gemcitabine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol, 22(1), 108-114. https://doi.org/10.1200/JCO.2004.06.068
22/1/108 [pii]
Kollmannsberger, C., Daneshmand, S., So, A., Chi, K. N., Murray, N., Moore, C., . . . Nichols, C. (2010). Management of disseminated nonseminomatous germ cell tumors with risk-based chemotherapy followed by response-guided postchemotherapy surgery. J Clin Oncol, 28(4), 537-542.
https://pubmed.ncbi.nlm.nih.gov/20026807/
10.1200/JCO.2009.23.0755
Kollmannsberger, C., Moore, C., Chi, K. N., Murray, N., Daneshmand, S., Gleave, M., . . . Nichols, C. R. (2010). Non-risk-adapted surveillance for patients with stage I nonseminomatous testicular germ-cell tumors: diminishing treatment-related morbidity while maintaining efficacy. Ann Oncol, 21(6), 1296-1301. https://doi.org/10.1093/annonc/mdp473
Kollmannsberger, C., Nichols, C., Meisner, C., Mayer, F., Kanz, L., & Bokemeyer, C. (2000). Identification of prognostic subgroups among patients with metastatic 'IGCCCG poor-prognosis' germ-cell cancer: an explorative analysis using cart modeling. Ann Oncol, 11(9), 1115-1120. https://doi.org/10.1023/a:1008333229936
Kollmannsberger, C., Tandstad, T., Bedard, P. L., Cohn-Cedermark, G., Chung, P. W., Jewett, M. A., . . . Nichols, C. R. (2015). Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol, 33(1), 51-57. https://doi.org/10.1200/JCO.2014.56.2116
Kollmannsberger, C., Tyldesley, S., Moore, C., Chi, K. N., Murray, N., Daneshmand, S., . . . Nichols, C. (2010). Evolution in management of testicular seminoma: population-based outcomes with selective utilization of active therapies. Ann Oncol.
https://pubmed.ncbi.nlm.nih.gov/20926549/
10.1093/annonc/mdq466
Kondagunta, G. V., Bacik, J., Donadio, A., Bajorin, D., Marion, S., Sheinfeld, J., . . . Motzer, R. J. (2005). Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol, 23(27), 6549-6555.
https://pubmed.ncbi.nlm.nih.gov/16170162/
10.1200/JCO.2005.19.638
Kvammen, O., Myklebust, T. A., Solberg, A., Moller, B., Klepp, O. H., Fossa, S. D., & Tandstad, T. (2016). Long-term Relative Survival after Diagnosis of Testicular Germ Cell Tumor. Cancer Epidemiol Biomarkers Prev, 25(5), 773-779. https://doi.org/10.1158/1055-9965.EPI-15-1153
Kvammen, O., Myklebust, T. A., Solberg, A., Moller, B., Klepp, O. H., Fossa, S. D., & Tandstad, T. (2019). Causes of inferior relative survival after testicular germ cell tumor diagnosed 1953-2015: A population-based prospective cohort study. PLoS One, 14(12), e0225942. https://doi.org/10.1371/journal.pone.0225942
Langberg, M. K., Berglund-Nord, C., Cohn-Cedermark, G., Haugnes, H. S., Tandstad, T., & Langberg, C. W. (2018). Imatinib may reduce chemotherapy-induced pneumonitis. A report on four cases from the SWENOTECA. Acta Oncol, 57(10), 1401-1406. https://doi.org/10.1080/0284186X.2018.1479072
Laughlin, G. A., Barrett-Connor, E., & Bergstrom, J. (2008). Low serum testosterone and mortality in older men. J Clin Endocrinol Metab, 93(1), 68-75. https://doi.org/10.1210/jc.2007-1792
Leibovitch, I., Baniel, J., Foster, R. S., & Donohue, J. P. (1995). The clinical implications of procedural deviations during orchiectomy for nonseminomatous testis cancer. J Urol, 154(3), 935-939.
Leu, L., & Baribeault, D. (2010). A comparison of the rates of cisplatin (cDDP)--induced nephrotoxicity associated with sodium loading or sodium loading with forced diuresis as a preventative measure. J Oncol Pharm Pract, 16(3), 167-171. https://doi.org/10.1177/1078155209346071
Lorch, A., Bascoul-Mollevi, C., Kramar, A., Einhorn, L., Necchi, A., Massard, C., . . . Beyer, J. (2011). Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol, 29(16), 2178-2184. https://doi.org/10.1200/JCO.2010.32.6678
Lorch, A., Beyer, J., Bascoul-Mollevi, C., Kramar, A., Einhorn, L. H., Necchi, A., . . . Kollmannsberger, C. K. (2010). Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol, 28(33), 4906-4911.
https://pubmed.ncbi.nlm.nih.gov/20956623/
10.1200/JCO.2009.26.8128
Lorch, A., Mollevi, A., Kramar, A., Einhorn, L. H., & Necchi, C. (2010). Conventional-dose versus high-dose chemotherapy in relapsed or refractory male germ-cell tumors: A retrospective study in 1,594 patients. J Clin Oncol, 28(15s), (suppl: abstr 4513).
Lotz, J. P., Bui, B., Gomez, F., Theodore, C., Caty, A., Fizazi, K., . . . Groupe d'Etudes des Tumeurs, U.-G. (2005). Sequential high-dose chemotherapy protocol for relapsed poor prognosis germ cell tumors combining two mobilization and cytoreductive treatments followed by three high-dose chemotherapy regimens supported by autologous stem cell transplantation. Results of the phase II multicentric TAXIF trial. Ann Oncol, 16(3), 411-418. https://doi.org/10.1093/annonc/mdi087
Lov om pasient- og brukerrettigheter (pasient- og brukerrettighetsloven). LOV-1999-07-02-63.
Luz, M. A., Kotb, A. F., Aldousari, S., Brimo, F., Tanguay, S., Kassouf, W., & Aprikian, A. G. (2010). Retroperitoneal lymph node dissection for residual masses after chemotherapy in nonseminomatous germ cell testicular tumor. World J Surg Oncol, 8, 97. https://doi.org/10.1186/1477-7819-8-97
Lyman, G. H., Bohlke, K., Falanga, A., & American Society of Clinical, O. (2015). Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract, 11(3), e442-444. https://doi.org/10.1200/JOP.2015.004473
Laaksonen, D. E., Niskanen, L., Punnonen, K., Nyyssonen, K., Tuomainen, T. P., Valkonen, V. P., . . . Salonen, J. T. (2004). Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care, 27(5), 1036-1041. https://doi.org/10.2337/diacare.27.5.1036
MacMahon, H., Naidich, D. P., Goo, J. M., Lee, K. S., Leung, A. N. C., Mayo, J. R., . . . Bankier, A. A. (2017). Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology, 284(1), 228-243. https://doi.org/10.1148/radiol.2017161659
Martin, W. G., Ristow, K. M., Habermann, T. M., Colgan, J. P., Witzig, T. E., & Ansell, S. M. (2005). Bleomycin pulmonary toxicity has a negative impact on the outcome of patients with Hodgkin's lymphoma. J Clin Oncol, 23(30), 7614-7620. https://doi.org/10.1200/JCO.2005.02.7243
Masterson, T. A., & Cary, C. (2018). The Use of Modified Templates in Early and Advanced Stage Nonseminomatous Germ Cell Tumor. Adv Urol, 2018, 6783147. https://doi.org/10.1155/2018/6783147
Masterson, T. A., Shayegan, B., Carver, B. S., Bajorin, D. F., Feldman, D. R., Motzer, R. J., . . . Sheinfeld, J. (2012). Clinical impact of residual extraretroperitoneal masses in patients with advanced nonseminomatous germ cell testicular cancer. Urology, 79(1), 156-159. https://doi.org/10.1016/j.urology.2011.09.038
McCaffrey, J. A., Mazumdar, M., Bajorin, D. F., Bosl, G. J., Vlamis, V., & Motzer, R. J. (1997). Ifosfamide- and cisplatin-containing chemotherapy as first-line salvage therapy in germ cell tumors: response and survival. J Clin Oncol, 15(7), 2559-2563.
McKenney, J. K., Heerema-McKenney, A., & Rouse, R. V. (2007). Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Advances in anatomic pathology, 14(2), 69-92. https://doi.org/10.1097/PAP.0b013e31803240e6
Measurements, I. I. C. o. R. U. a. (1999). Prescribing, recording and reporting photon beam therapy (Supplement to ICRU Report 50). ICRU Report 62. Oxford University Press, Oxford, United Kingdom
Mezvrishvili, Z., & Managadze, L. (2006). Retroperitoneal lymph node dissection for high-risk stage I and stage IIA seminoma. Int Urol Nephrol, 38(3-4), 615-619. https://doi.org/10.1007/s11255-005-4793-x
Miller, J. C., & Einhorn, L. H. (1990). Phase II study of daily oral etoposide in refractory germ cell tumors. Semin Oncol, 17(1 Suppl 2), 36-39.
Mineur, P., De Cooman, S., Hustin, J., Verhoeven, G., & De Hertogh, R. (1987). Feminizing testicular Leydig cell tumor: hormonal profile before and after unilateral orchidectomy. J Clin Endocrinol Metab, 64(4), 686-691. https://doi.org/10.1210/jcem-64-4-686
Moch, H., Cubilla, A. L., Humphrey, P. A., Reuter, V. E., & Ulbright, T. M. (2016). The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol, 70(1), 93-105. https://doi.org/10.1016/j.eururo.2016.02.029
Moller, H., & Skakkebaek, N. E. (1999). Risk of testicular cancer in subfertile men: case-control study. BMJ, 318(7183), 559-562.
Mooney, K. L., & Kao, C. S. (2018). A Contemporary Review of Common Adult Non-germ Cell Tumors of the Testis and Paratestis. Surg Pathol Clin, 11(4), 739-758. https://doi.org/10.1016/j.path.2018.07.002
Mosharafa, A. A., Foster, R. S., Leibovich, B. C., Bihrle, R., Johnson, C., & Donohue, J. P. (2003). Is post-chemotherapy resection of seminomatous elements associated with higher acute morbidity? J Urol, 169(6), 2126-2128. https://doi.org/10.1097/01.ju.0000060121.33899.4b
Motzer, R. J., Nichols, C. J., Margolin, K. A., Bacik, J., Richardson, P. G., Vogelzang, N. J., . . . Bosl, G. J. (2007). Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol, 25(3), 247-256. https://pubmed.ncbi.nlm.nih.gov/17235042/
10.1200/JCO.2005.05.4528
Murphy, B. R., Breeden, E. S., Donohue, J. P., Messemer, J., Walsh, W., Roth, B. J., & Einhorn, L. H. (1993). Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol, 11(2), 324-329. https://doi.org/10.1200/JCO.1993.11.2.324
Necchi, A., Lo Vullo, S., Giannatempo, P., Raggi, D., Calareso, G., Togliardi, E., . . . Salvioni, R. (2017). Pazopanib in advanced germ cell tumors after chemotherapy failure: results of the open-label, single-arm, phase 2 Pazotest trial. Ann Oncol, 28(6), 1346-1351. https://doi.org/10.1093/annonc/mdx124
Nichols, C. R. (1999). Treatment of recurrent germ cell tumors. Semin Surg Oncol, 17(4), 268-274. https://pubmed.ncbi.nlm.nih.gov/10588856/
Nichols, C. R., Roth, B., Albers, P., Einhorn, L. H., Foster, R., Daneshmand, S., . . . Kollmannsberger, C. (2013). Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol, 31(28), 3490-3493. https://doi.org/10.1200/JCO.2012.47.6010
Nicolai, N., Necchi, A., Gianni, L., Piva, L., Biasoni, D., Torelli, T., . . . Salvioni, R. (2009). Long-term results of a combination of paclitaxel, cisplatin and gemcitabine for salvage therapy in male germ-cell tumours. BJU Int, 104(3), 340-346.
https://pubmed.ncbi.nlm.nih.gov/19239440/
10.1111/j.1464-410X.2009.08453.x
Nini, A., Konieczny, M., Winter, C., Lusch, A., Krauspe, R., & Albers, P. (2018). Surgical management and outcomes of patients with bone metastases in germ cell tumors: A case series. Urol Oncol, 36(2), 82 e81-82 e85. https://doi.org/10.1016/j.urolonc.2017.10.016
Nonomura, N., Nagahara, A., Oka, D., Mukai, M., Nakai, Y., Nakayama, M., . . . Miki, T. (2009). Brain metastases from testicular germ cell tumors: a retrospective analysis. International journal of urology : official journal of the Japanese Urological Association, 16(11), 887-893. https://doi.org/10.1111/j.1442-2042.2009.02391.x
Nord, C., Bjoro, T., Ellingsen, D., Mykletun, A., Dahl, O., Klepp, O., . . . Fossa, S. D. (2003). Gonadal hormones in long-term survivors 10 years after treatment for unilateral testicular cancer. Eur Urol, 44(3), 322-328. https://doi.org/10.1016/s0302-2838(03)00263-x
Norway, C. R. o. (2019). Cancer in Norway 2018 - Cancer incidence, mortality, survival and prevalence in Norway.
Nuver, J., Smit, A. J., Sleijfer, D. T., van Gessel, A. I., van Roon, A. M., van der Meer, J., . . . Gietema, J. A. (2004). Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer, 40(5), 701-706. https://doi.org/10.1016/j.ejca.2003.12.012
Nuver, J., Smit, A. J., Wolffenbuttel, B. H., Sluiter, W. J., Hoekstra, H. J., Sleijfer, D. T., & Gietema, J. A. (2005). The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol, 23(16), 3718-3725.
https://pubmed.ncbi.nlm.nih.gov/15738540/
10.1200/JCO.2005.02.176
O'Sullivan, J. M., Huddart, R. A., Norman, A. R., Nicholls, J., Dearnaley, D. P., & Horwich, A. (2003). Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol, 14(1), 91-96.
Oechsle, K., & Bokemeyer, C. (2011). Treatment of brain metastases from germ cell tumors. Hematol Oncol Clin North Am, 25(3), 605-613, ix. https://doi.org/10.1016/j.hoc.2011.03.012
Oechsle, K., Hartmann, M., Mehnert, A., Oing, C., Bokemeyer, C., & Vehling, S. (2016). Symptom burden in long-term germ cell tumor survivors. Support Care Cancer, 24(5), 2243-2250. https://doi.org/10.1007/s00520-015-3026-9
Oing, C., Giannatempo, P., Honecker, F., Oechsle, K., Bokemeyer, C., & Beyer, J. (2018). Palliative treatment of germ cell cancer. Cancer Treat Rev, 71, 102-107. https://doi.org/10.1016/j.ctrv.2018.10.007
Oing, C., Oechsle, K., Necchi, A., Loriot, Y., De Giorgi, U., Flechon, A., . . . Bokemeyer, C. (2014). Bone metastases in germ cell tumors: Results from an international data base. J Clin Oncol, (32), Supple; abstr 4559.
Oing, C., Oechsle, K., Necchi, A., Loriot, Y., De Giorgi, U., Flechon, A., . . . Bokemeyer, C. (2017). Impact of primary metastatic bone disease in germ cell tumors: results of an International Global Germ Cell Tumor Collaborative Group G3 Registry Study. Ann Oncol, 28(3), 576-582. https://doi.org/10.1093/annonc/mdw648
Oldenburg, J., Lorch, A., & Fossa, S. D. (2011). Late relapse of germ cell tumors. Hematol Oncol Clin North Am, 25(3), 615-626, x.
https://pubmed.ncbi.nlm.nih.gov/21570613/
10.1016/j.hoc.2011.03.006
Oldenburg, J., Wahlqvist, R., & Fossa, S. D. (2009). Late relapse of germ cell tumors. World Journal of Urology, 27(4), 493-500. https://doi.org/10.1007/s00345-009-0411-3
Oliver, R. T., Mason, M. D., Mead, G. M., von der Maase, H., Rustin, G. J., Joffe, J. K., . . . Stenning, S. P. (2005). Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet, 366(9482), 293-300.
https://pubmed.ncbi.nlm.nih.gov/16039331/
10.1016/S0140-6736(05)66984-X
Oliver, R. T., Mead, G. M., Rustin, G. J., Joffe, J. K., Aass, N., Coleman, R., . . . Stenning, S. P. (2011). Randomized Trial of Carboplatin Versus Radiotherapy for Stage I Seminoma: Mature Results on Relapse and Contralateral Testis Cancer Rates in MRC TE19/EORTC 30982 Study (ISRCTN27163214). J Clin Oncol, 29(8), 957-962.
https://pubmed.ncbi.nlm.nih.gov/21282539/
10.1200/JCO.2009.26.4655
Olofsson, S. E., Tandstad, T., Jerkeman, M., Dahl, O., Stahl, O., Klepp, O., . . . Cavallin-Stahl, E. (2011). Population-based study of treatment guided by tumor marker decline in patients with metastatic nonseminomatous germ cell tumor: a report from the Swedish-Norwegian Testicular Cancer Group. J Clin Oncol, 29(15), 2032-2039. https://doi.org/10.1200/JCO.2010.29.1278
Paffenholz, P., Grein, K., Heidegger, I., Nestler, T., Grabbert, M., Salem, J., . . . Heidenreich, A. (2019). Predictors of thrombosis in testicular cancer during platinum-based chemotherapy. World Journal of Urology, 37(9), 1907-1916. https://doi.org/10.1007/s00345-018-2598-7
Paffenholz, P., Pfister, D., & Heidenreich, A. (2020). Testis-preserving strategies in testicular germ cell tumors and germ cell neoplasia in situ. Transl Androl Urol, 9(Suppl 1), S24-S30. https://doi.org/10.21037/tau.2019.07.22
Patel, H. D., Singla, N., Ghandour, R. A., Freifeld, Y., Cheaib, J. G., Woldu, S. L., . . . Bagrodia, A. (2019). Site of extranodal metastasis impacts survival in patients with testicular germ cell tumors. Cancer, 125(22), 3947-3952. https://doi.org/10.1002/cncr.32427
Pearce, S. M., Golan, S., Gorin, M. A., Luckenbaugh, A. N., Williams, S. B., Ward, J. F., . . . Eggener, S. E. (2017). Safety and Early Oncologic Effectiveness of Primary Robotic Retroperitoneal Lymph Node Dissection for Nonseminomatous Germ Cell Testicular Cancer. Eur Urol, 71(3), 476-482. https://doi.org/10.1016/j.eururo.2016.05.017
Pectasides, D., Pectasides, M., Farmakis, D., Aravantinos, G., Nikolaou, M., Koumpou, M., . . . Raptis, S. A. (2004). Oxaliplatin and irinotecan plus granulocyte-colony stimulating factor as third-line treatment in relapsed or cisplatin-refractory germ-cell tumor patients: a phase II study. Eur Urol, 46(2), 216-221. https://doi.org/10.1016/j.eururo.2004.03.001
S030228380400106X [pii]
Pectasides, D., Pectasides, M., Farmakis, D., Aravantinos, G., Nikolaou, M., Koumpou, M., . . . Skarlos, D. (2004). Gemcitabine and oxaliplatin (GEMOX) in patients with cisplatin-refractory germ cell tumors: a phase II study. Ann Oncol, 15(3), 493-497.
Pettus, J. A., Carver, B. S., Masterson, T., Stasi, J., & Sheinfeld, J. (2009). Preservation of ejaculation in patients undergoing nerve-sparing postchemotherapy retroperitoneal lymph node dissection for metastatic testicular cancer. Urology, 73(2), 328-331; discussion 331-322. https://doi.org/10.1016/j.urology.2008.08.501
Pico, J. L., Rosti, G., Kramar, A., Wandt, H., Koza, V., Salvioni, R., . . . Biron, P. (2005). A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol, 16(7), 1152-1159.
https://pubmed.ncbi.nlm.nih.gov/15928070/
10.1093/annonc/mdi228
Pierorazio, P. M., & Biles, M. J. (2019). Indications for Surgery in Disseminated Seminoma. Urol Clin North Am, 46(3), 399-407. https://doi.org/10.1016/j.ucl.2019.04.005
Pont, J., Albrecht, W., Postner, G., Sellner, F., Angel, K., & Holtl, W. (1996). Adjuvant chemotherapy for high-risk clinical stage I nonseminomatous testicular germ cell cancer: long-term results of a prospective trial. J Clin Oncol, 14(2), 441-448.
Puc, H. S., Heelan, R., Mazumdar, M., Herr, H., Scheinfeld, J., Vlamis, V., . . . Motzer, R. J. (1996). Management of residual mass in advanced seminoma: results and recommendations from the Memorial Sloan-Kettering Cancer Center. J Clin Oncol, 14(2), 454-460.
Quek, M. L., Simma-Chiang, V., Stein, J. P., Pinski, J., Quinn, D. I., & Skinner, D. G. (2005). Postchemotherapy residual masses in advanced seminoma: current management and outcomes. Expert Rev Anticancer Ther, 5(5), 869-874. https://doi.org/10.1586/14737140.5.5.869
Raggi, D., Giannatempo, P., Miceli, R., Fare, E., Piva, L., Biasoni, D., . . . Necchi, A. (2017). Etoposide, Methotrexate, and Dactinomycin Alternating With Cyclophosphamide and Vincristine (EMACO) for Male Patients With HCG-expressing, Chemoresistant Germ Cell Tumors. Am J Clin Oncol, 40(1), 60-65. https://doi.org/10.1097/COC.0000000000000113
Ravi, R., Ong, J., Oliver, R. T., Badenoch, D. F., Fowler, C. G., & Hendry, W. F. (1999). The management of residual masses after chemotherapy in metastatic seminoma. BJU Int, 83(6), 649-653.
Risk, M. C., & Foster, R. S. (2011). Postchemotherapy retroperitoneal lymph node dissection for testis cancer. Expert Rev Anticancer Ther, 11(1), 95-106. https://doi.org/10.1586/era.10.206
Roth, L. M., & Cheng, L. (2018). Classical gonadoblastoma: its relationship to the 'dissecting' variant and undifferentiated gonadal tissue. Histopathology, 72(4), 545-555. https://doi.org/10.1111/his.13387
Ruf, C. G., Sanatgar, N., Isbarn, H., Ruf, B., Simon, J., Fankhauser, C. D., & Dieckmann, K. P. (2020). Leydig-cell tumour of the testis: retrospective analysis of clinical and therapeutic features in 204 cases. World Journal of Urology. https://doi.org/10.1007/s00345-020-03079-1
Rutgers, J. L., Young, R. H., & Scully, R. E. (1988). The testicular "tumor" of the adrenogenital syndrome. A report of six cases and review of the literature on testicular masses in patients with adrenocortical disorders. Am J Surg Pathol, 12(7), 503-513.
Sakaguchi, Y., & Isowa, N. (2012). Successful resection of mediastinal seminoma evaluated the response to induction chemotherapy with fluorodeoxyglucose-positron emission tomography. Ann Thorac Cardiovasc Surg, 18(1), 45-47. https://doi.org/10.5761/atcs.cr.11.01667
Sawrie, S. M., Guthrie, B. L., Spencer, S. A., Nordal, R. A., Meredith, R. F., Markert, J. M., . . . Fiveash, J. B. (2008). Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys, 70(1), 181-186. https://doi.org/10.1016/j.ijrobp.2007.05.084
Saxman, S. B., Nichols, C. R., & Einhorn, L. H. (1997). Pulmonary toxicity in patients with advanced-stage germ cell tumors receiving bleomycin with and without granulocyte colony stimulating factor. Chest, 111(3), 657-660. https://doi.org/10.1378/chest.111.3.657
Schirren, J., Trainer, S., Eberlein, M., Lorch, A., Beyer, J., & Bolukbas, S. (2012). The role of residual tumor resection in the management of nonseminomatous germ cell cancer of testicular origin. Thorac Cardiovasc Surg, 60(6), 405-412. https://doi.org/10.1055/s-0031-1299584
Schmoll, H. J. (2002). Extragonadal germ cell tumors. Ann Oncol, 13 Suppl 4, 265-272. https://doi.org/10.1093/annonc/mdf669
Schmoll, H. J., Kollmannsberger, C., Metzner, B., Hartmann, J. T., Schleucher, N., Schoffski, P., . . . German Testicular Cancer Study, G. (2003). Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group. J Clin Oncol, 21(22), 4083-4091. https://doi.org/10.1200/JCO.2003.09.035
Schneider, D. T., Schuster, A. E., Fritsch, M. K., Hu, J., Olson, T., Lauer, S., . . . Perlman, E. J. (2001). Multipoint imprinting analysis indicates a common precursor cell for gonadal and nongonadal pediatric germ cell tumors. Cancer Res, 61(19), 7268-7276.
Scholz, M., Zehender, M., Thalmann, G. N., Borner, M., Thoni, H., & Studer, U. E. (2002). Extragonadal retroperitoneal germ cell tumor: evidence of origin in the testis. Ann Oncol, 13(1), 121-124. https://doi.org/10.1093/annonc/mdf003
Schultz, S. M., Einhorn, L. H., Conces, D. J., Jr., Williams, S. D., & Loehrer, P. J. (1989). Management of postchemotherapy residual mass in patients with advanced seminoma: Indiana University experience. J Clin Oncol, 7(10), 1497-1503.
Sharmeen, F., Rosenthal, M. H., & Howard, S. A. (2014). The management of retroperitoneal lymphadenopathy in spermatocytic seminoma of the testicle. Clin Imaging, 38(2), 202-204. https://doi.org/10.1016/j.clinimag.2013.11.006
Sharp, D. S., Carver, B. S., Eggener, S. E., Kondagunta, G. V., Motzer, R. J., Bosl, G. J., & Sheinfeld, J. (2008). Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol, 26(34), 5524-5529.
https://pubmed.ncbi.nlm.nih.gov/18936477/
10.1200/JCO.2007.15.7453
Shores, M. M., Matsumoto, A. M., Sloan, K. L., & Kivlahan, D. R. (2006). Low serum testosterone and mortality in male veterans. Arch Intern Med, 166(15), 1660-1665. https://doi.org/10.1001/archinte.166.15.1660
Simone, C. B., 2nd, Kramer, K., O'Meara, W. P., Bekelman, J. E., Belard, A., McDonough, J., & O'Connell, J. (2012). Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys, 82(1), 242-249. https://doi.org/10.1016/j.ijrobp.2010.11.021
Skoogh, J., Steineck, G., Cavallin-Stahl, E., Wilderang, U., Hakansson, U. K., Johansson, B., . . . Swenoteca. (2011). Feelings of loss and uneasiness or shame after removal of a testicle by orchidectomy: a population-based long-term follow-up of testicular cancer survivors. International Journal of Andrology, 34(2), 183-192.
https://doi.org/10.1111/J.1365-2605.2010.01073.X
Sleijfer, S. (2001). Bleomycin-induced pneumonitis. Chest, 120(2), 617-624.
Smith, E. M., Pang, H., Cirrincione, C., Fleishman, S., Paskett, E. D., Ahles, T., . . . Alliance for Clinical Trials in, O. (2013). Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA, 309(13), 1359-1367. https://doi.org/10.1001/jama.2013.2813
Socialstyrelsen. (2019). Cancer Incidence in Sweden 2018.
Sohaib, S. A., Koh, D. M., Barbachano, Y., Parikh, J., Husband, J. E., Dearnaley, D. P., . . . Huddart, R. (2009). Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin Radiol, 64(4), 362-367. https://doi.org/10.1016/j.crad.2008.10.011
Spermon, J. R., De Geus-Oei, L. F., Kiemeney, L. A., Witjes, J. A., & Oyen, W. J. (2002). The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int, 89(6), 549-556.
Spiess, P. E., Brown, G. A., Liu, P., Tannir, N. M., Tu, S. M., Evans, J. G., . . . Pisters, L. L. (2006). Predictors of outcome in patients undergoing postchemotherapy retroperitoneal lymph node dissection for testicular cancer. Cancer, 107(7), 1483-1490. https://doi.org/10.1002/cncr.22182
Sprauten, M., Haugnes, H. S., Brydoy, M., Kiserud, C., Tandstad, T., Bjoro, T., . . . Oldenburg, J. (2015). Chronic fatigue in 812 testicular cancer survivors during long-term follow-up: increasing prevalence and risk factors. Ann Oncol, 26(10), 2133-2140. https://doi.org/10.1093/annonc/mdv328
Srikanthan, A., Tran, B., Beausoleil, M., Jewett, M. A., Hamilton, R. J., Sturgeon, J. F., . . . Bedard, P. L. (2015). Large retroperitoneal lymphadenopathy as a predictor of venous thromboembolism in patients with disseminated germ cell tumors treated with chemotherapy. J Clin Oncol, 33(6), 582-587. https://doi.org/10.1200/JCO.2014.58.6537
Stang, A., Trabert, B., Wentzensen, N., Cook, M. B., Rusner, C., Oosterhuis, J. W., & McGlynn, K. A. (2012). Gonadal and extragonadal germ cell tumours in the United States, 1973-2007. Int J Androl, 35(4), 616-625. https://doi.org/10.1111/j.1365-2605.2011.01245.x
Steiner, H., Holtl, L., Wirtenberger, W., Berger, A. P., Bartsch, G., & Hobisch, A. (2002). Long-term experience with carboplatin monotherapy for clinical stage I seminoma: a retrospective single-center study. Urology, 60(2), 324-328.
Steiner, H., Scheiber, K., Berger, A. P., Rein, P., Hobisch, A., Aufderklamm, J., . . . Zangerl, F. (2011). Retrospective multicentre study of carboplatin monotherapy for clinical stage I seminoma. BJU Int, 107(7), 1074-1079. https://doi.org/10.1111/j.1464-410X.2010.09658.x
Stepanian, S., Patel, M., & Porter, J. (2016). Robot-assisted Laparoscopic Retroperitoneal Lymph Node Dissection for Testicular Cancer: Evolution of the Technique. Eur Urol, 70(4), 661-667. https://doi.org/10.1016/j.eururo.2016.03.031
Stephenson, A. J., Bosl, G. J., Motzer, R. J., Bajorin, D. F., Stasi, J. P., & Sheinfeld, J. (2007). Nonrandomized comparison of primary chemotherapy and retroperitoneal lymph node dissection for clinical stage IIA and IIB nonseminomatous germ cell testicular cancer. J Clin Oncol, 25(35), 5597-5602. https://doi.org/10.1200/JCO.2007.12.0808
Sturgeon, J. F., Moore, M. J., Kakiashvili, D. M., Duran, I., Anson-Cartwright, L. C., Berthold, D. R., . . . Jewett, M. A. (2010). Non-Risk-Adapted Surveillance in Clinical Stage I Nonseminomatous Germ Cell Tumors: The Princess Margaret Hospital's Experience. Eur Urol.
https://pubmed.ncbi.nlm.nih.gov/21190791/
10.1016/j.eururo.2010.12.010
Svartberg, J., von Muhlen, D., Schirmer, H., Barrett-Connor, E., Sundfjord, J., & Jorde, R. (2004). Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol, 150(1), 65-71. https://doi.org/10.1530/eje.0.1500065
Tandstad, T., Cavallin-Stahl, E., Dahl, O., Haugnes, H. S., Langberg, C., Laurell, A., . . . Cohn-Cedermark, G. (2014). Management of clinical stage I seminomatous testicular cancer: A report from SWENOTECA. J Clin Oncol, 32(5s), suppl; abstract 4508.
Tandstad, T., Dahl, O., Cohn-Cedermark, G., Cavallin-Stahl, E., Stierner, U., Solberg, A., . . . Klepp, O. (2009). Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. J Clin Oncol, 27(13), 2122-2128.
https://pubmed.ncbi.nlm.nih.gov/19307506/
10.1200/JCO.2008.18.8953
Tandstad, T., Smaaland, R., Solberg, A., Bremnes, R. M., Langberg, C. W., Laurell, A., . . . Cohn-Cedermark, G. (2011). Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish norwegian testicular cancer study group. J Clin Oncol, 29(6), 719-725.
https://pubmed.ncbi.nlm.nih.gov/21205748/
10.1200/JCO.2010.30.1044
Tandstad, T., Stahl, O., Dahl, O., Haugnes, H. S., Hakansson, U., Karlsdottir, A., . . . Swenoteca. (2016). Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy, a report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA). Ann Oncol. https://doi.org/10.1093/annonc/mdw164
Tandstad, T., Stahl, O., Hakansson, U., Dahl, O., Haugnes, H. S., Klepp, O. H., . . . Swenoteca. (2014). One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol, 25(11), 2167-2172. https://doi.org/10.1093/annonc/mdu375
Thompson, R. H., Carver, B. S., Bosl, G. J., Bajorin, D., Motzer, R., Feldman, D., . . . Sheinfeld, J. (2010). Evaluation of lymph node counts in primary retroperitoneal lymph node dissection. Cancer, 116(22), 5243-5250. https://doi.org/10.1002/cncr.25266
Thorsen, L., Haugnes HS, Fosså SD, Brydoy M, Tandstad T, Wisløff T, Gjerset GM, Edvardsen E, Larsen K, Sandset PM, Henriksson CA, Raastad T, Negaard H. (2020). Thromboembolic events after high-intensity training during cisplatin-based chemotherapy for testicular cancer: Case reports and review of the literature. Int J Cancer, In press.
Tookman, L., Rashid, S., Matakidou, A., Phillips, M., Wilson, P., Ansell, W., . . . Shamash, J. (2013). Carboplatin AUC 10 for IGCCCG good prognosis metastatic seminoma. Acta Oncol, 52(5), 987-993. https://doi.org/10.3109/0284186X.2012.714078
Trama, A., Mallone, S., Nicolai, N., Necchi, A., Schaapveld, M., Gietema, J., . . . Group, R. W. (2012). Burden of testicular, paratesticular and extragonadal germ cell tumours in Europe. Eur J Cancer, 48(2), 159-169. https://doi.org/10.1016/j.ejca.2011.08.020
Tryakin, A., Fedyanin, M., Kanagavel, D., Fainstein, I., Sergeev, J., Polockij, B., . . . Tjulandin, S. (2011). Paclitaxel+BEP (T-BEP) regimen as induction chemotherapy in poor prognosis patients with nonseminomatous germ cell tumors: a phase II study. Urology, 78(3), 620-625. https://doi.org/10.1016/j.urology.2011.05.005
Ueno, T., Tanaka, Y. O., Nagata, M., Tsunoda, H., Anno, I., Ishikawa, S., . . . Itai, Y. (2004). Spectrum of germ cell tumors: from head to toe. Radiographics, 24(2), 387-404. https://doi.org/10.1148/rg.242035082
Utz, D. C., & Buscemi, M. F. (1971). Extragonadal testicular tumors. J Urol, 105(2), 271-274. https://doi.org/10.1016/s0022-5347(17)61508-8
van den Belt-Dusebout, A. W., Nuver, J., de Wit, R., Gietema, J. A., ten Bokkel Huinink, W. W., Rodrigus, P. T., . . . van Leeuwen, F. E. (2006). Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol, 24(3), 467-475.
https://ascopubs.org/doi/10.1200/JCO.2005.02.7193?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
10.1200/JCO.2005.02.7193
van Dijk, M. R., Steyerberg, E. W., & Habbema, J. D. (2006). Survival of non-seminomatous germ cell cancer patients according to the IGCC classification: An update based on meta-analysis. Eur J Cancer, 42(7), 820-826.
https://pubmed.ncbi.nlm.nih.gov/16574403/
10.1016/j.ejca.2005.08.043
Vaughn, D. J., Hwang, W. T., Lal, P., Rosen, M. A., Gallagher, M., & O'Dwyer, P. J. (2015). Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer, 121(9), 1463-1468. https://doi.org/10.1002/cncr.29213
Vuky, J., Bains, M., Bacik, J., Higgins, G., Bajorin, D. F., Mazumdar, M., . . . Motzer, R. J. (2001). Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising from the mediastinum. J Clin Oncol, 19(3), 682-688. https://doi.org/10.1200/JCO.2001.19.3.682
Warde, P., Specht, L., Horwich, A., Oliver, T., Panzarella, T., Gospodarowicz, M., & von der Maase, H. (2002). Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol, 20(22), 4448-4452.
Weibring, K., Nord, C., Stahl, O., Eberhard, J., Sandberg, K., Johansson, H., . . . Cohn-Cedermark, G. (2019). Sperm count in Swedish clinical stage I testicular cancer patients following adjuvant treatment. Ann Oncol, 30(4), 604-611. https://doi.org/10.1093/annonc/mdz017
Weijl, N. I., Rutten, M. F., Zwinderman, A. H., Keizer, H. J., Nooy, M. A., Rosendaal, F. R., . . . Osanto, S. (2000). Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. J Clin Oncol, 18(10), 2169-2178. https://doi.org/10.1200/JCO.2000.18.10.2169
Weissbach, L., Bussar-Maatz, R., & Mann, K. (1997). The value of tumor markers in testicular seminomas. Results of a prospective multicenter study. Eur Urol, 32(1), 16-22.
Wethal, T., Kjekshus, J., Roislien, J., Ueland, T., Andreassen, A. K., Wergeland, R., . . . Fossa, S. D. (2007). Treatment-related differences in cardiovascular risk factors in long-term survivors of testicular cancer. J Cancer Surviv, 1(1), 8-16. https://doi.org/10.1007/s11764-007-0012-3
Widen, S. E., & Erlandsson, S. I. (2004). Self-reported tinnitus and noise sensitivity among adolescents in Sweden. Noise Health, 7(25), 29-40.
Wierecky, J., Kollmannsberger, C., Boehlke, I., Kuczyk, M., Schleicher, J., Schleucher, N., . . . Bokemeyer, C. (2005). Secondary leukemia after first-line high-dose chemotherapy for patients with advanced germ cell cancer. J Cancer Res Clin Oncol, 131(4), 255-260. https://doi.org/10.1007/s00432-004-0628-x
Wilder, R. B., Buyyounouski, M. K., Efstathiou, J. A., & Beard, C. J. (2012). Radiotherapy treatment planning for testicular seminoma. Int J Radiat Oncol Biol Phys, 83(4), e445-452. https://doi.org/10.1016/j.ijrobp.2012.01.044
Zagars, G. K., Ballo, M. T., Lee, A. K., & Strom, S. S. (2004). Mortality after cure of testicular seminoma. J Clin Oncol, 22(4), 640-647. https://doi.org/10.1200/JCO.2004.05.205
JCO.2004.05.205 [pii]
Zhang, M., Kao, C. S., Ulbright, T. M., & Epstein, J. I. (2013). Testicular fibrothecoma: a morphologic and immunohistochemical study of 16 cases. Am J Surg Pathol, 37(8), 1208-1214. https://doi.org/10.1097/PAS.0b013e318286c129
Zhou, E. S., Hall, K. T., Michaud, A. L., Blackmon, J. E., Partridge, A. H., & Recklitis, C. J. (2019). Open-label placebo reduces fatigue in cancer survivors: a randomized trial. Support Care Cancer, 27(6), 2179-2187. https://doi.org/10.1007/s00520-018-4477-6
Zilli, T., Boudreau, C., Doucet, R., Alizadeh, M., Lambert, C., van Nguyen, T., & Taussky, D. (2011). Bone marrow-sparing intensity-modulated radiation therapy for Stage I seminoma. Acta Oncol, 50(4), 555-562. https://doi.org/10.3109/0284186x.2011.564650